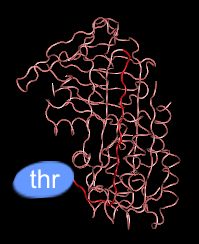
In plasma: slightly inhibitory
As antithrombin III circulates in the plasma, the loop that interacts with thrombin (shown in red) is not fully extended. This conformation is only a weak inhibitor of thrombin.

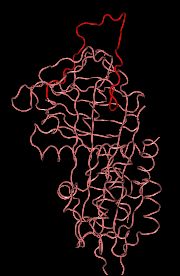
Bound to heparin on the vessel wall: highly inhibitory
Heparin is a carbohydrate that is attached to the surface of the cells that line blood vessels.
Heparin binds to antithrombin III at a site that is far away from the loop that inhibits thrombin. Heparin induces an allosteric transition in antithrombin that fully extends the inhibitory loop, greatly increasing the loop's affinity for thrombin.
By binding to heparin, the highly active form of antithrombin III becomes teathered to the surface of intact blood vessels.
Analogues of heparin are commonly used in medical practice as anticoagulants. These heparin analogues work as a anticoagulants by activating antithrombin III.
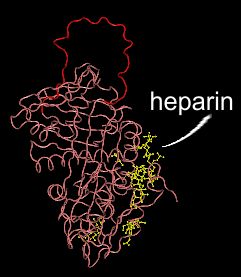
After cleavage by thrombin: Inactive complex with thrombin.
After antithrombin III's inhibitory loop is cleaved it folds into antithrombin III's seconadry structure, forming part of a pleated sheet. This causes a change in the heparin binding site, releasing the complex from the vessel wall.
Thrombin, which remains attached to antithrombin III after the antithrombin III is cleaved, is represented by the blue circle in this picture.