INTRODUCTION
Click The Image
A Partial Roadmap For Amino Acid Metabolism
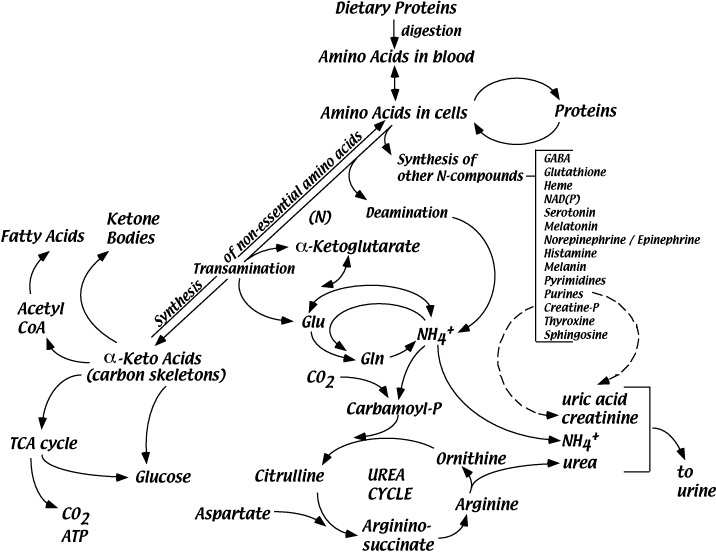
Blood Provides A Pool Of Amino Acids For Use By Cells
There are no storage molecules for amino acids as there are for carbohydrates, i.e., glucose in glycogen, or for fatty acids, as in triacylglycerols (fats). The body maintains a relatively large free amino acid pool in the blood (approximately 35-65 mg/deciLiter), even during fasting; cells and tissues have continuous access to individual amino acids for the synthesis of proteins and essential amino acid derivatives. The blood concentrations of two amino acids, alanine and glutamine, which serve special purposes, are higher than those of the other amino acids.
Processes That Use Amino Acids
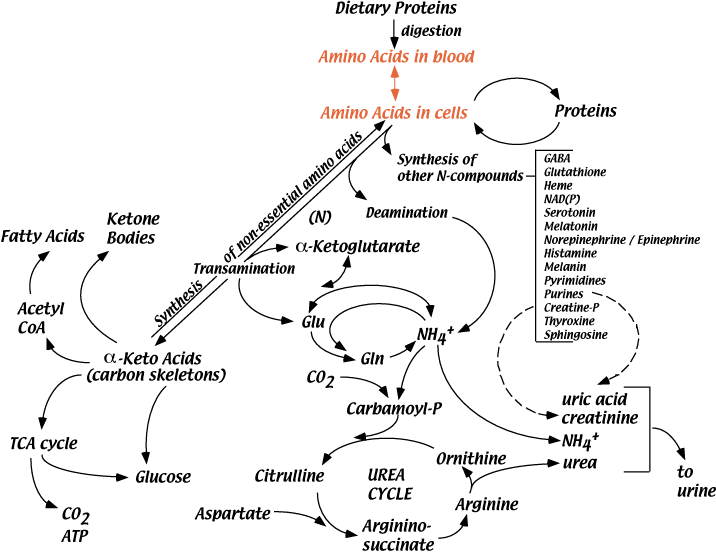
Protein Synthesis
Proteins in the body are constantly synthesized and degraded, partially draining and refilling the cellular amino acid pools. In a well fed, healthy human adult, approximately 300 - 600 grams of new protein are synthesized each day. Growth factors, hormones, including insulin, and cytokines stimulate protein synthesis.
Processes That Use Amino Acids
Synthesis Of Other Nitrogen-Containing Molecules
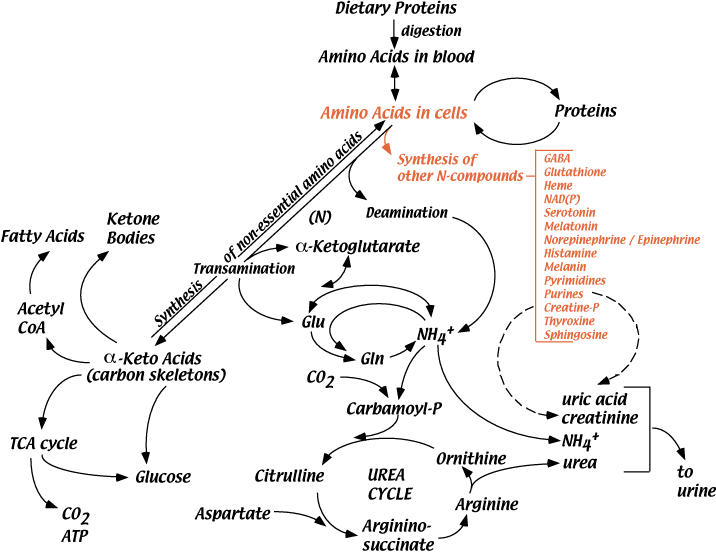
All the useful nitrogen in the body is supplied by amino acids. All nitrogen-containing compounds synthesized in the body derive their nitrogen from amino acids — cellular proteins, hormones (e.g., thyroxine, epinephrine, insulin), neurotransmitters, creatine phosphate, heme in hemoglobin and cytochromes, melanin, purine and pyrimidine bases. This is only a partial list of all the nitrogen-containing compounds that derive their nitrogen from amino acids.
Synthesis Of Other Nitrogen-Containing Molecules
An Example — Creatinine
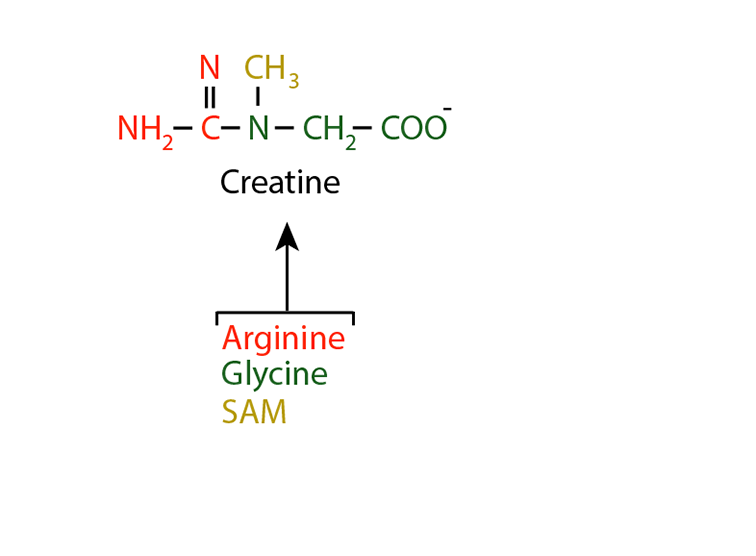
Creatinine is a nitrogen-containing molecule synthesized predominantly in muscles. Creatine is synthesized first from argining, glycine and S-adenosyl methionine (SAM).
Synthesis Of Other Nitrogen-Containing Molecules
An Example — Creatinine
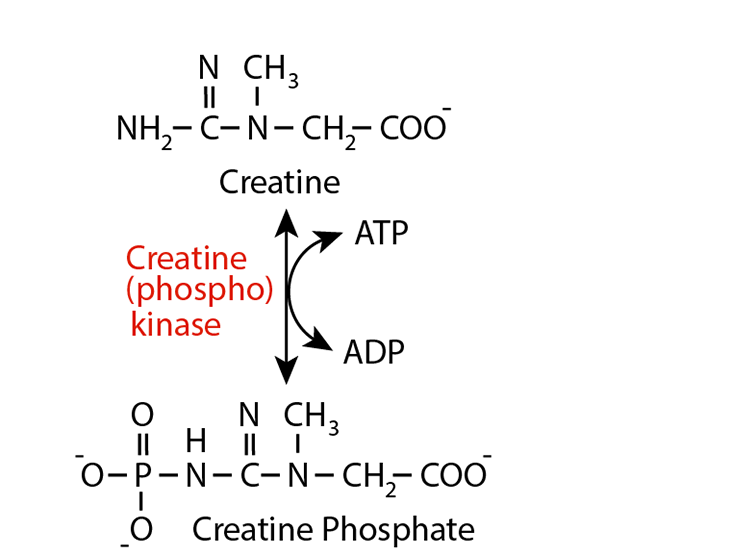
Creatine (phospho) kinase converts creatine to creatine phosphate, which accumulates in muscle cells as an energy buffer when ATP is aboundant. Its phosphate group is readily donated to ADP, thereby boosting the ATP content of the muscle celln as they hydrolyse ATP for energy to derive muscle contraction.
Synthesis Of Other Nitrogen-Containing Molecules
An Example — Creatinine
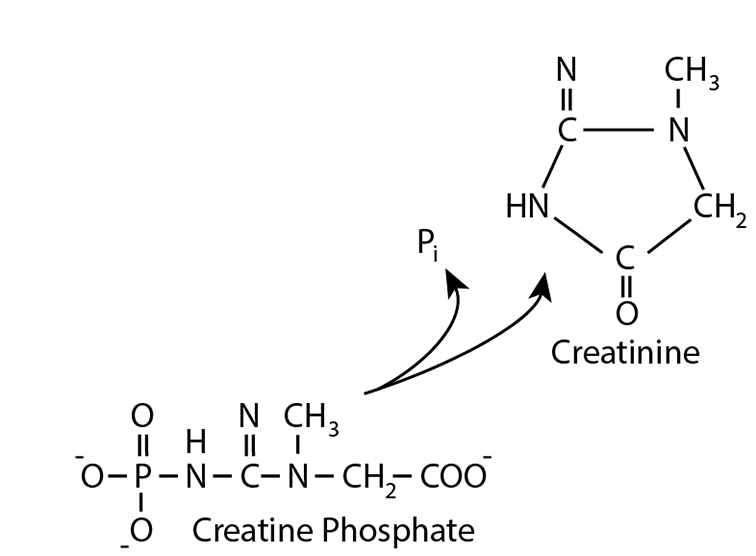
Creatine phosphate is spontaneously (non-enzymatically) dephosphorylated, resulting in the cyclization of the dephosphorylated molecule to yield creatinine, which ...
Synthesis Of Other Nitrogen-Containing Molecules
An Example — Creatinine
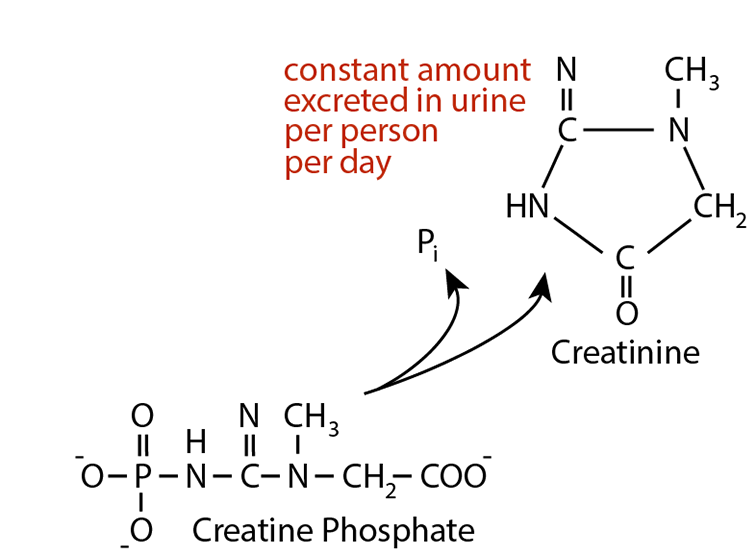
is readily excreted in the urine. The amount of creatinine produced is proportional to the muscle mass and is released from muscle at a constant rate. The amount excreted in the urine per day per person is constant and independent of the volume of urine excreted. Elevataed creatinine in the blood relates to impaired kidney function, i.e., impared Glomerular Flow Rate (GFR). Creatinine clearance rate (C
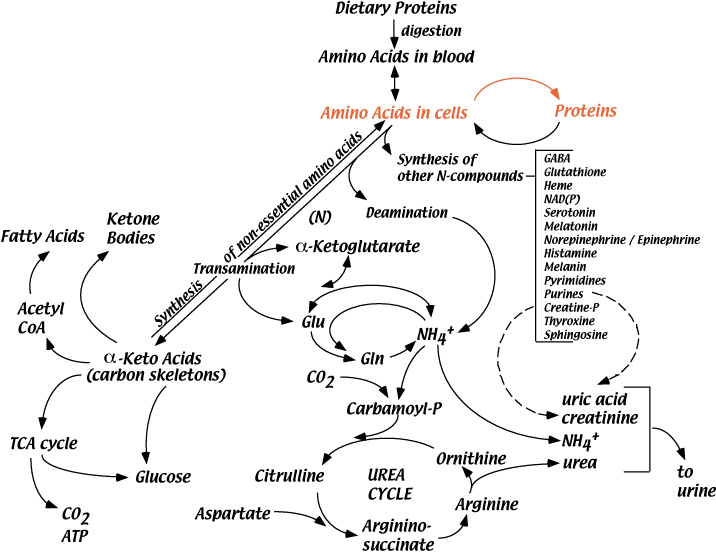
Processes That Use Amino Acids
Degradation Of Amino Acids
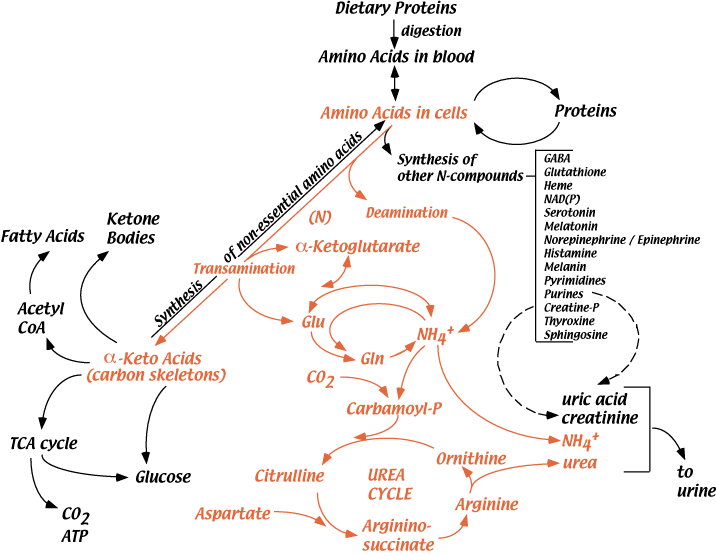
Amino acids are continuously degraded. Their nitrogen is removed either by deamination or by transamination reactions that donate it to various α-keto acids (see “Nitrogen” in the top menu). Ultimately, the nitrogen is excreted, mainly as urea, but also as NH4+ or other nitrogen-containing compounds. Normally, urea accounts for about 90% of all excreted nitrogen. Amino acid carbon skeletins are reused for the synthesis of other molecules, are a major source of carbon skeletons for the synthesis of glucose (gluconeogenesis) or are oxidized for the production of energy.
Processes That Contribute Amino Acids
Dietary Protein
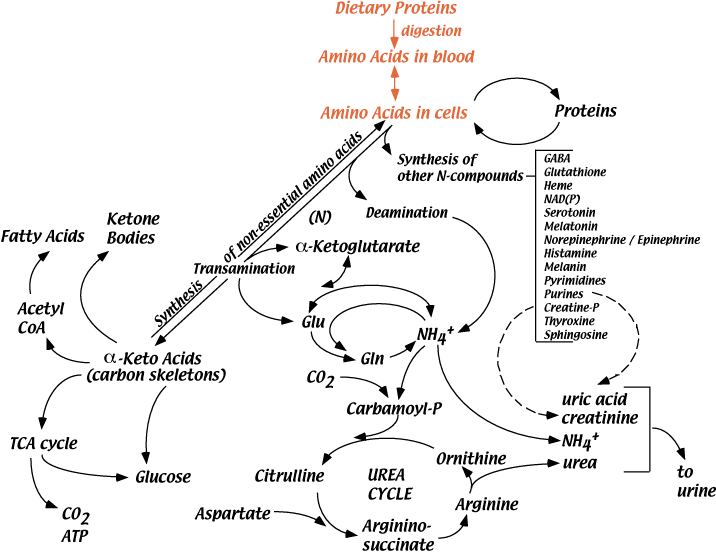
Average adult humans require approximately 60 - 100 grams of dietary protein per day. Amino acids are produced by digestion of dietary proteins in the intestines, absorbed through the intestinal epithelial cells, and enter the blood. Various cells take up these amino acids, which enter the cellular amino acid pools. Amino acids are used for the synthesis of proteins and other nitrogen-containing compounds, or their carbon skeletons are oxidized for energy or the synthesis of glucose.
Processes That Contribute Amino Acids
Dietary Protein — Failure To Injest Adequate Protein: Kwashiorkor
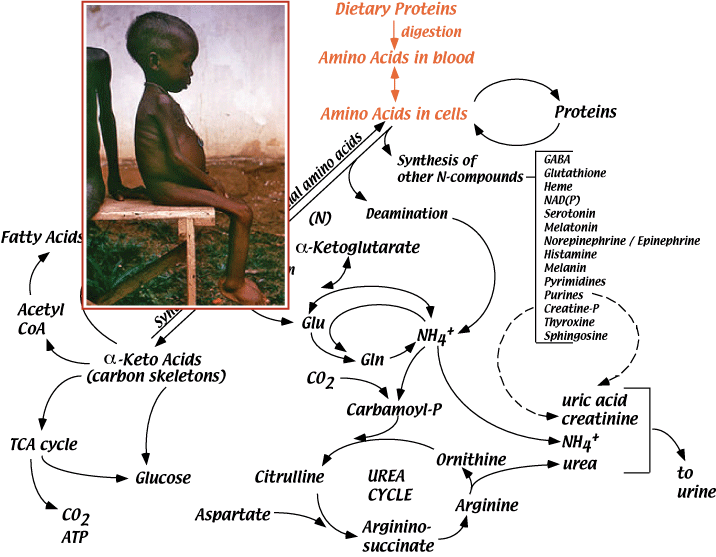
Failure to injest sufficient protein results in kwashiokor. Kwashiorkor is a state of malnutrition that results from a deficiency of dietary protein in the presence of a normal or high carbohydrate intake. Kwashiorkor is most common between the ages of 1 and 4 years, but can occur in infancy. There are many causes of kwashiorkor, but weaning is the major factor, when breast milk is replaced by an inadequate and often unbalanced diet. Infants are most frequently affected in times of famine, when their mother is also starved for protein. Kwashiorkor symptoms may develop slowly over time. Common symptoms include: abdominal swelling, distension or bloating, diarrhea, enlarged, fatty liver, fatigue, frequent infections, generalized swelling, hair and nail changes, including brittle, reddish hair and ridged nails that are thin and soft, Irritability, muscle wasting, skin changes, including pigment loss, red or purple patches, peeling, cracking, skin sloughing, and the development of sores, slowed growth leading to short stature, weight loss. Treatment is protein supplementation often in the form of dried skim milk.
Processes That Contribute Amino Acids
Protein Degradation
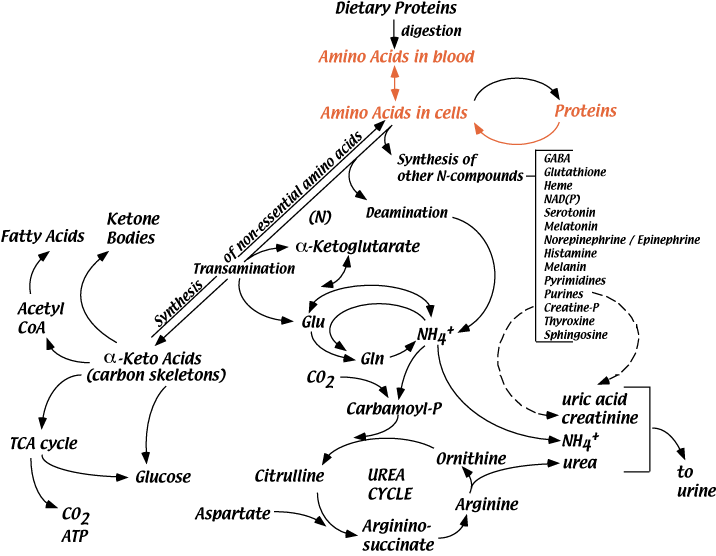
In a well fed, healthy human adult, approximately 300 - 600 grams of protein are degraded to amino acids each day. Normally, this degradation is balanced by the synthesis of 300 - 600 grams of protein per day. Protein turnover allows changes in the quantities of different proteins produced as physiology requires, and removes modified or damaged proteins. Decreased insulin shifts the balance between protein synthesis and protein degradation toward degradation, resulting in a net loss of protein. During some “chronic stresses” cellular proteins are degraded to provide amino acids for functions that help alleviate the stress (see “Hypothelamic-Pituitary-Adrenal Axis” below).
Processes That Contribute Amino Acids
Synthesis Of Non-Essential Amino Acids
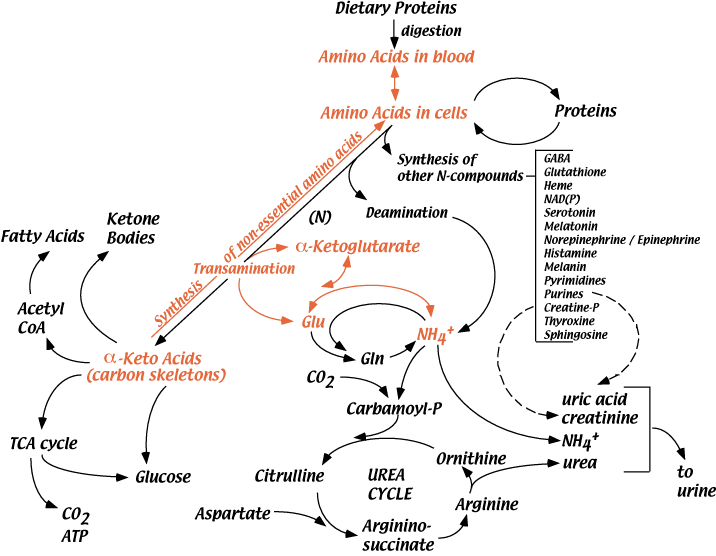
Humans can synthesize 10 of the 20 common amino acids — the Non-essential Amino Acids. The remaining 10 common amino acids — the Essential AMino Acids — must be taken in the diet.
Processes That Contribute Amino Acids
Synthesis Of Non-Essential Amino Acids — The Essential Amino Acids
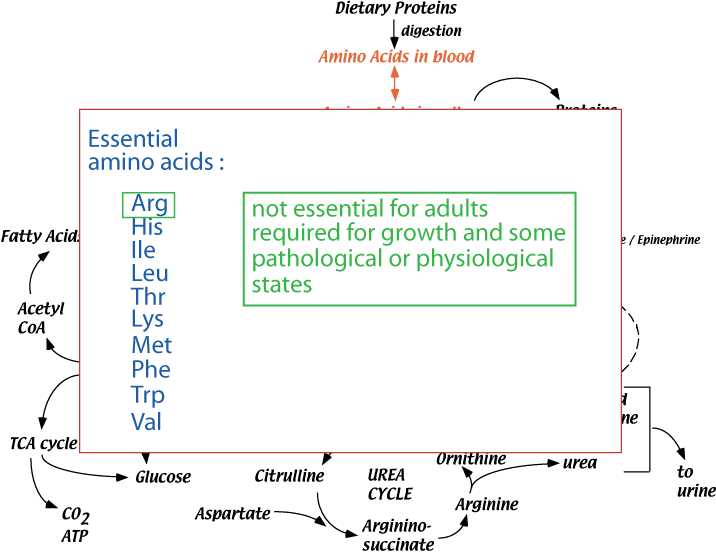
Two amino acids that are normally non-essential in healthy adults — arginine and histidine — are not synthesized in sufficient quantities to allow normal growth of children and adolescents and are, therefore, essential for these individuals, and also in some pathological or physiological states when increased protein synthesis is required. All the other common amino acids are non-essential. Lack of a single essential amino acid halts protein synthesis and causes the other excess, unused amino acids to be degraded.
Nitrogen Balance
Nitrogen balance is the difference between the amount of nitrogen taken into the body (mainly as dietary protein) and the amount lost in urine, sweat, feces.
Nitrogen Balance
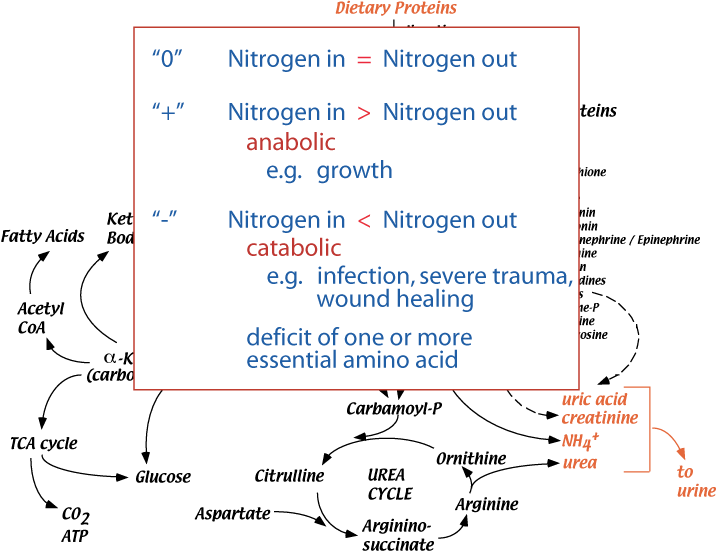
Healthy adult humans are in nitrogen balance — Zero nitrogen balance: nitrogen intake = nitrogen excreted (mainly as urea in the urine). Positive nitrogen balance: nitrogen intake is greater than nitrogen excreted. Positive nitrogen balance results primarily when new tissue is produced (e.g., during body growth in childhood and adolescence, during pregnancy, and during major wound healing, as after major surgery). Negative nitrogen balance: nitrogen intake is less than nitrogen excreted. Negative nitrogen balance occurs when digestion of body protein exceeds synthesis, and results from several circumstances, e.g., too little dietary protein. too little of one or more of the essential amino acids in the diet, certain hypercatolytic states.
Nitrogen Balance
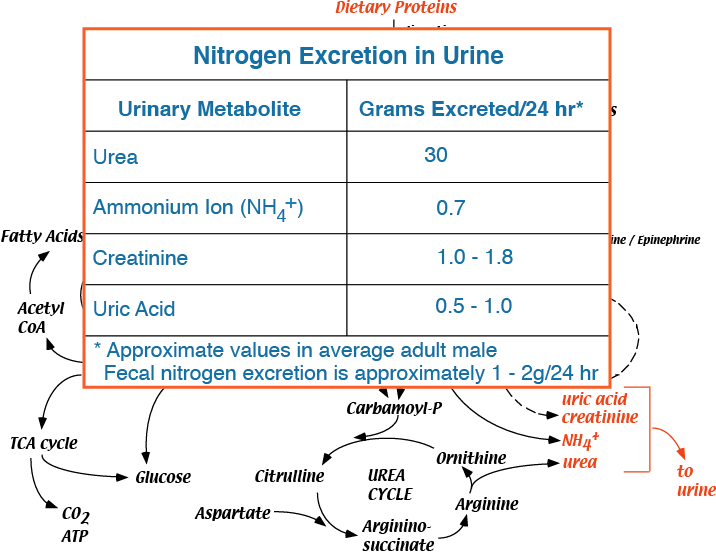
Major urinary nitrogen excretory products
- Average adult humans require approximately 60 -100 grams of dietary protein per day.
- Amino acids are produced by digestion of dietary proteins in the intestines, absorbed through the intestinal epithelial cells, and enter the blood.
- Various cells take up these amino acids, which enter the cellular amino acid pools.
- Amino acids are used for the synthesis of proteins and other nitrogen-containing compounds, or their carbon skeletons are oxidized for energy or used for the synthesis of glucose during hypoglycemia.
- The body maintains a relatively large free amino acid pool in the blood (approximately 35-65 mg/deciLiter), even during fasting; tissues have continuous access to individual amino acids for the synthesis of proteins and essential amino acid derivatives, such as neurotransmitters. The amino acid pool also provides the liver with substrates for gluconeogenesis and ketogenesis. The free amino acid pool is derived from dietary amino acids and the proteolysis of body proteins.
- All useful nitrogen in the body is derived from amino acids.
- All nitrogen-containing compounds of the body are synthesized from amino acids - cellular proteins, hormones (e.g., thyroxine, epinephrine, insulin), neurotransmitters, creatine phosphate, heme in hemoglobin and cytochromes, melanin, purine and pyrimidine bases.
- Proteins in the body are constantly synthesized and degraded, partially draining and refilling the cellular amino acid pools.
- In a well fed human adult, approximately 300 - 600 grams of protein are degraded, and approximately 300 - 600 grams of new protein are synthesized each day.
- Protein turnover allows shifts in the quantities of different proteins produced as physiology requires, and removes modified or damaged proteins.
- In muscle, during fasting, or other stresses, the synthesis/degradation equilibrium is shifted toward degradation, resulting in loss of muscle mass. The resulting amino acids can be released into the blood for conversion to glucose by the liver to supply metabolic energy for critical tissues (e.g., red blood cells and brain) or to supply amino acids to tissues that respond to a particular stress.
- Insulin promotes protein synthesis by muscle, and decreased blood insulin levels, during fasting for example, result in net proteolysis and release of amino acids from muscle into the blood.
-
Cortisol, the major chronic stress hormone, is a glucocorticoid released in response to various stresses that induces the degradation of proteins — see the “Hypothelamic-Pituitary-Adrenal Axis”. The resulting amino acids can be used for several different purposes to counteract the effects of particular chronic stresses, e.g., for the synthesis of proteins in the proliferation of cells of the the immune system to respond to infection or for new cells to repair damaged tissues. Alternatively, the amino acids can be degraded to provide carbon skeletons as an energy source or for the synthesis of glucose. In acidosis, the amino and amide groups of glutamine are used by the kidney to buffer excess protons for excretion in the urine (see “AA Flux” in the top menu).
Click The Image
Hypothelamic-Pituitary-Adrenal Axis
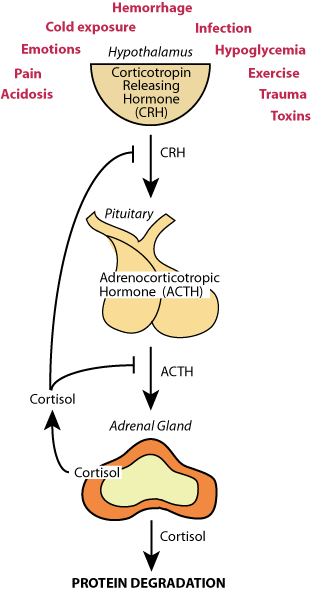
Chronic stresses, acidosis, pain, hypoglycemia, etc., induce the hypothalamus to release corticotropin releasing hormone (CRH), which acts on the pituitary to cause the release of adrenocorticotropic hormone (ACTH), which acts on the adrenal gland to cause the secretion of cortisol, the major chronic stress hormone. In a feed-back loop, cortisol inhibits the release of CRH and ACTH.
Hypothelamic-Pituitary-Adrenal Axis
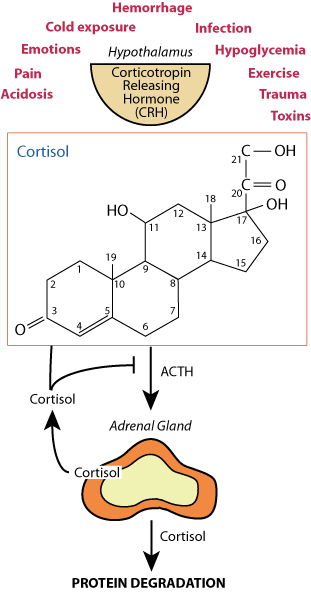
Chorisol is a steriod hormone, whose action is to induce the transcription of genes that are the targets of the glucacorticoid nuclear hormone receptor, some of which encode or activate proteases.
Hypothelamic-Pituitary-Adrenal Axis
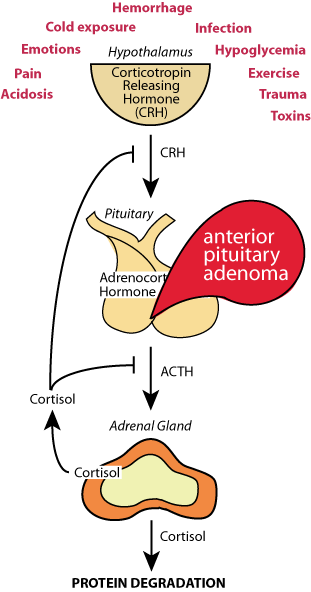
ACTH-secreting adenomas of the anterior pituitary gland can cause excessive amounts of cortisol to be secreted by the adrenal cortex. The cortisol inhibition of ACTH secretion fails, leading to excessive tissue protein degradation, resulting in muscle wasting — Cushing's disease.
Hypothelamic-Pituitary-Adrenal Axis
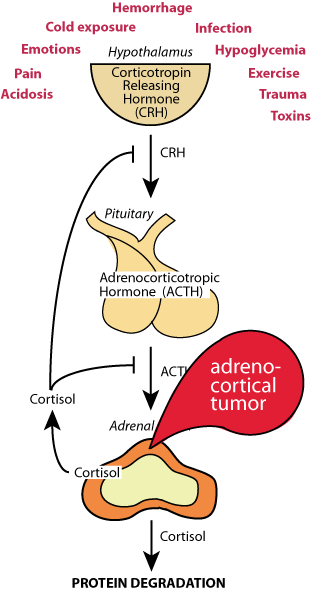
Tumors of the cortisol-secreting cells of the adrenal cortex secrete excessive cortisol, resulting in excessive tissue protein degradation and muscle wasting — Cushing's syndrome
- In a well fed human adult, approximately 300 - 600 grams of protein are degraded, and approximately 300 - 600 grams of new protein are synthesized each day.
- As a source of energy, amino acid carbon skeletons are directly oxidized, or, in the starved state, converted to glucose and ketone bodies, and then oxidized.
- Nitrogen must be removed before the carbon skeletons of amino acids are oxidized.
- The liver is the major site of amino acid oxidation, but most tissues can oxidize the branched chain amino acids (i.e., leucine, isoleucine, valine).
- Most of the carbons from amino acid degradation are converted to pyruvate, intermediates of the TCA cycle or acetyl CoA. During fasting these carbons are converted to glucose in the liver and kidney, or to ketone bodies in the liver. In the well fed state, they may be used for lipogenesis.
- Amino acid nitrogen forms ammonia, which is toxic.
- The liver is the major site of amino acid metabolism in the body and the major site of urea synthesis. The liver is also the major site of amino acid degradation, and partially oxidizes most amino acids, converting the carbon skeleton to glucose, ketone bodies, or CO2. In liver, the urea cycle converts ammonia and the amino groups from amino acids to urea (see “Nitrogen > Urea Cycle” in the top menu), which is non-toxic, water-soluble, and easily excreted in the urine.
- Nitrogen derived from amino acid catabolism in other tissues is transported to the liver, in large part, as alanine or glutamine, the major transporters of ammonia in the blood.
- Certain physiological states trigger protein breakdown to generate amino acids as a source of energy. Skeletal muscle, the largest tissue contributor to the body’s amino acid pool derived from protein breakdown, uses branched chain amino acids particularly well as an energy source. Nitrogen derived from these, and other amino acids, is converted in skeletal muscle mainly to alanine and glutamine, which account for approximately 50% of total α-amino nitrogen released by skeletal muscle.
- Alanine, a transamination product (see “Nitrogen > Nitrogen Reactions” in the top menu) of its cognate α-keto acid, pyruvate, can donate its amino group via transamination in the liver, and its carbon skeleton can be oxidized for energy derivation, or converted to glucose via the gluconeogenesis pathway for export to the blood and use by other tissues (the so-called “alanine / glucose” cycle &mdash see “AA Flux” in the top menu).
- Glucagon enhances alanine transport into the liver. This makes physiological sense because glucagon signals that the blood glucose level is low, a condition to which skeletal muscle responds by increasing protein breakdown to yield amino acid carbon skeletons as an energy source. Excess nitrogen derived from the increased amino acid pool must be disposed of, first by transport to the liver, in large part as alanine, and then converted, in the liver, to urea for excretion. Increased transport of alanine into the liver, promoted by glucagon, helps the body dispose of the excess nitrogen, and supplies the liver with carbon skeletons for glucose synthesis — the alanine / glucose cycle (see “AA Flux” in the top menu).
- Glutamine released from skeletal muscle and other tissues serves several functions:
- In kidney the nitrogen carried by glutamine is released and excreted into the urine, allowing removal, as NH4+, of protons formed during fuel oxidation, thereby helping maintain the body’s pH, especially during metabolic acidosis, when other methods of buffering excess protons may become exceeded.
- Glutamine provides a fuel source for the kidney.
- In rapidly dividing cells (e.g., lymphocytes and macrophages), glutamine is used as a fuel, as a nitrogen donor for biosynthetic reactions, and as substrate for protein synthesis. During sepsis, for example, increased numbers of lymphocytes and macrophages are required to subdue infection. Muscle protein breakdown increases to help provide energy and amino acids for the synthesis of proteins and othere nitrogen-containing compounds needed to produce these cells.
- The Non-essential amino acids
- Twelve amino acids present in proteins are synthesized in the body - eleven (serine, glycine, cysteine, alanine, aspartate, asparagine, glutamate, glutamine, proline, arginine, histidine) are produced from glucose carbon skeletons, one (tyrosine) is produced from phenylalanine.
- The Essential amino acids
- Ten amino acids present in proteins (arginine, histidine, isoleucine, leucine, threonine, lysine, methionine, phenylalanine, tryptophan, valine) are required in the diet of a growing human.
- Arginine, although not required in the diets of adults, is required for growth (children and adolescents), because the amounts that can be synthesized are not sufficient to maintain normal growth rates.
- Larger amounts of phenylalanine are required if the diet is low in tyrosine because tyrosine is synthesized from phenylalanine. Larger amounts of methionine are required if the diet is low in cysteine because the sulfur of methionine is donated for the synthesis of cysteine.
- Nitrogen balance is the difference between the amount of nitrogen taken into the body (mainly as dietary protein) and the amount lost mainly in urine and to a lesser extent in feces and sweat.
- Proteins of the body are constantly being degraded to amino acids and resynthesized — some proteins have long half-lives, while others have short half-lives. Free amino acids can have two fates: either they are used for synthesis of proteins and other essential nitrogen-containing compounds, or their carbon skeletons are oxidized as fuel to yield energy and during hypoglycemia converted to glucose. When amino acid carbon skeletons are oxidized for energy or converted to glucose their nitrogen atoms are excreted in the urine, principally in the form of urea (see “Nitrogen > Urea Cycle” in the top menu).
- Healthy adult humans are in nitrogen balance (sometimes referred to as Zero nitrogen balance): nitrogen intake = nitrogen excreted (mainly as urea in the urine)
- Positive nitrogen balance — nitrogen intake > nitrogen excreted: Positive nitrogen balance results primarily when new tissue is produced (e.g., during body growth in childhood and adolescence, during pregnancy, and during major wound healing, as after major surgery).
- Negative nitrogen balance — nitrogen intake < nitrogen excreted: Negative nitrogen balance occurs when excess amino acids are metabolized producing increased ammonia and ammonium ion, and can result from several circumstances:
- insufficient dietary protein
- deficit of one or more of the essential amino acids in the diet
Because all 20 amino acids are required for protein synthesis to proceed, a deficit of any one amino acid reduces or prevents protein synthesis, and the use of the other amino acids for protein synthesis is reduced or abolished. Excess amino acids, both from protein degradation in the absence protein synthesis — a disruption of the balnace between protein synthesis and protein degradation — and dietary input are degraded, resulting in an increase in ammonia and ammonium ion, which are converted to urea for disposal. - Trauma, burns, and septic stress are examples of hypercatabolic states characterized by increased fuel utilization and negative nitrogen balance. In these hypercatabolic states, skeletal muscle protein synthesis decreases and protein degradation increases in an attempt to supply the body with carbon skeletons for energy derivation, or amino acids to repair body damage. The negative nitrogen balance that occurs in these hypercatabolic states results from the accelerated net protein degradation, producing amino acids that must be deaminated before their carbon skeletons can be used as an energy source. The resulting, excess ammonia and ammonium ion are disposed of as urea.
- If negative nitrogen balance persists for too long, body function is impaired because of the net loss of critical proteins
- The dominant end product of nitrogen metabolism in humans is urea.
- Amino acids in excess of the quantities needed for the synthesis of protein and other nitrogen containing metabolites are neither stored nor excreted. Rather, virtually all amino acid nitrogen is excreted in the form of urea and NH4+. On an average diet, an adult human excretes approximately 25 to 30 grams of urea per day, which represents approximately 90% of the total nitrogenous substances in the urine.