GLUCONEOGENESIS
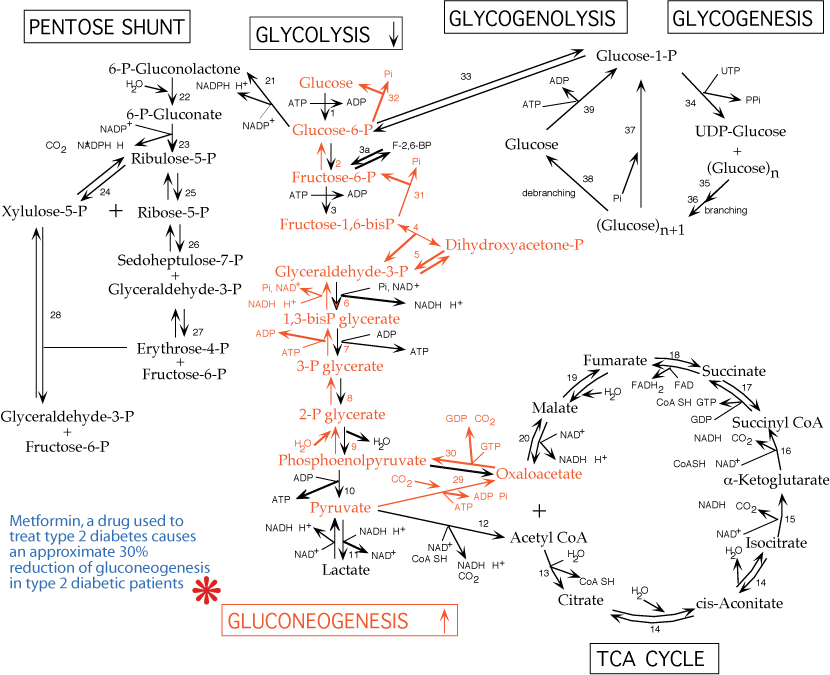 V. Gluconeogenesis is NOT glycolysis run backwards
V. Gluconeogenesis is NOT glycolysis run backwards
The daily glucose requirement for a typical adult is about 160 - 190 grams, 120 - 140 grams of which are used by the brain, which depends on a continual glucose supply. Red blood cells also require glucose as a fuel (anaerobic glycolysis) because they lack mitochondria and are, therefore, unable to use other fuel molecules. The blood contains about 20 grams of glucose and approximately 190 grams are stored as glycogen, for a total reserve lasting about one day. During fasting/starvation glucose must be supplied from non-carbohydrate sources such as lactate, amino acids from muscle protein breakdown and glycerol from adipose fat breakdown.
Six high energy bonds and two NADH are expended to synthesize one molecule of glucose from 2 molecules of pyruvate. Synthesized glucose is usually for export from the liver into the blood during fasting.
The reversible reactions of the glycolysis pathway are used for gluconeogenesis, but the irreversible reactions require other reactions to bypass them. These irreversible reactions and those that bypass them are the major sites of regulation of both pathways.
- Carboxylation of pyruvate to form oxaloacetate by pyruvate carboxylase:

- irreversible
- allosteric activation by acetyl CoA
- inhibited by ADP
- mitochondrial localization
- ATP is expended
- The vitamin biotin is the required CO2 (obtained from HCO3-) carrier/donor
- Oxaloacetate is transferred to the cytosol as malate, where it is regenerated by a cytosolic malate dehydrogenase.
- Transfer of a high-energy phosphate bond to oxaloacetate to form phosphoenolpyruvate by phosphoenolpyruvate carboxykinase [PEPCK]:

- inhibited by ADP
- transcription of the gene for phosphoenolpyruvate carboxykinase is increased by increased glucagon signaling and decreased insulin signaling in response to low blood [glucose]
- GTP is expended

In the liver, pyruvate kinase is phosphorylated by protein kinase A (cyclic AMP-dependent protein kinase), which becomes activated in response to increased glucagon signaling and decreased insulin signaling when blood [glucose] is reduced. This phosphroylation causes a reduction in the activity of pyruvate kinase. Consequently, the conversion of pyruvate to phosphoenolpyruvate, first to oxaloacetate by pyruvate carboxylase and then from oxaloacetate to phosphoenolpyruvate by phosphoenolpyruvate carboxykinase, predominates over the conversion of phosphoenolpyruvate to pyruvate by pyruvate kinase.Click The Image
PEPCK Gene Regulation: Increased Glucose

At normal or increased blood glucose, insulin signaling activates protein kinase B/AKT [PKB/AKT], which phosphorylates transcription factor FOXO1, causing it to be sequestered in the cytosol by its binding partner, a 14-3-3 protein. The transcription factor CREB is not phosphorylated and not active.PEPCK Gene Regulation: Decreased Glucose

When the blood glucose level falls, the pancreatic α cells secrete glucagon and the β cells stop secreting insulin. Cellular phosphatases remove the phosphate groups from PKB/AKT and FOXO1 releases from the 14-3-3 protein. CREB is phosphorylated by protein kinase A (cAMP-dependent protein kinase) upon its activation by the glucagon signaling pathway.PEPCK Gene Regulation: Decreased Glucose

Unphosphorylated FOXO1 enters the nucleus andPEPCK Gene Regulation: Decreased Glucose

binds to the Insulin Response Element (IRE) upstream of the PEPCK gene. Transcription is activated.
The two enzymatic activities of the liver bifunctional enzyme, Phosphofructokinase 2/Fructose 2,6-bisPhosphatase, regulate [Fructose 2,6-bisPhosphate] in response to blood [glucose]. Phosphorylation of the liver bifunctional enzyme by protein kinase A (cyclic AMP-dependent protein kinase) in response to increased glucagon signaling and decreased insulin signaling when blood [glucose] is reduced results in an increase in Fructose 2,6-bisPhosphatse activity and a decrease in Phosphofructokinase 2 activity, thereby reducing [Fructose 2,6-bisPhosphate]. Absent Fructose 2,6-bisPhosphate, the activity of Phosphofructokinase 1 is reduced and Fructose 1,6-bisPhosphatase activity prevails. - Dephosphorylation of fructose-1,6-bisphosphate to form fructose-6-phosphate by fructose 1,6-bisphosphatase:

- inhibited by fructose-6-phosphate, AMP and fructose-2,6-bisphosphate
- stimulated by citrate
- transcription of the gene for fructose 1,6-bisphosphatase is increased by increased glucagon signaling and decreased insulin signaling in response to low blood [glucose]
- Dephosphorylation of glucose-6-phosphate to form glucose by glucose-6-phosphatase:

- membrane-bound in the endoplasmic reticulum
- present only in liver and kidney, the two tissues that can export glucose into the blood.
- Transcription of the glucose-6-phosphatase gene is up regulated in response to glucagon signaling and down regulated in response to insulin signaling.
 Glucose 6-phosphatase activity is present at the inner suface of the endoplasmic reticulum (ER) in liver and kidney; other tissues are devoid of glucose 6-phosphatase. Glucose 6-phosphate is transported into the ER lumen by a transporter protein. Glucose 6-phosphatase catalyses the hydrolysis of the phosphate, yielding glucose and inorganic phosphate (Pi), which are transported out of the ER lumen to the cytosol respectively by Transporter 2 and Transporter 3. An essential stabilizing protein is associated with the glucose 6-phosphatase.
Glucose 6-phosphatase activity is present at the inner suface of the endoplasmic reticulum (ER) in liver and kidney; other tissues are devoid of glucose 6-phosphatase. Glucose 6-phosphate is transported into the ER lumen by a transporter protein. Glucose 6-phosphatase catalyses the hydrolysis of the phosphate, yielding glucose and inorganic phosphate (Pi), which are transported out of the ER lumen to the cytosol respectively by Transporter 2 and Transporter 3. An essential stabilizing protein is associated with the glucose 6-phosphatase.

Glucokinase gene transcription is repressed by increased glucagon signaling and decreased insulin sigling in response to low blood [glucose]. Also, when blood [glucose] is low, existing glucokinase enzyme is bound by the Glucokinase Regulatory Protein [GKRP], which inactivates and sequesters it in the cell nucleus, thereby preventing the glucose produced by gluconeogenesis (and glycogenolysis) from being re-phosphorylated and retained in the liver.
Summary of gluconeogenesis:

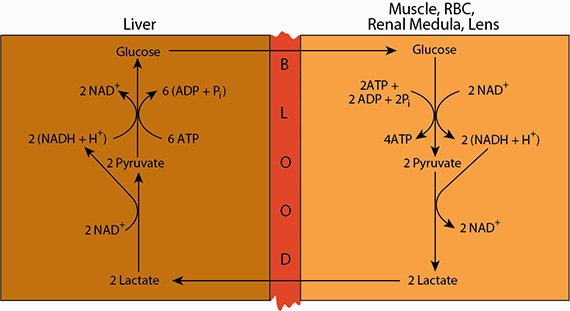 Cori Cycle: Lactate produced in extrahepatic tissue is transported via the blood to the liver, where it is oxidized to pyruvate, which is coverted to oxaloacetate by Pyruvate Carboxylase (reaction 29), and subsequently to glucose for export from the liver.
Cori Cycle: Lactate produced in extrahepatic tissue is transported via the blood to the liver, where it is oxidized to pyruvate, which is coverted to oxaloacetate by Pyruvate Carboxylase (reaction 29), and subsequently to glucose for export from the liver. Lactate produced by various tissues that undergo anaerobic glycolysis is transported to other tissues — mainly liver, heart and skeletal muscle — and oxidized to pyruvate. In the liver, the pyruvate sereves as one of the major sources of carbon skeletons for the synthesis of glucose, which is returned to the blood. This cycling of lactate and glucose between extrahepatic tissues and the liver is named the “Cori Cycle” after Carl and Gerty Cory, who elucidated it.
Eighteen of the common 20 Amino Acids are Glucogenic (i.e., supply carbon skeletons for the synthesis of glucose). Except for the three branched chain amino acids (leucine, isoleucine, valine) the liver is the major site of amino acid degradation; the liver obtains a substantial amount of its energy requirement from the degradation of amino acids.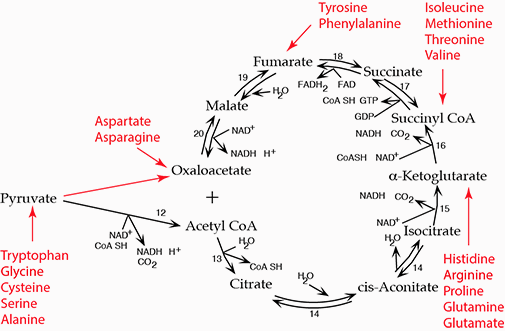 Carbon skeletons from the degration of amino acids either enter the TCA cycle or are converted to pyruvate.
Carbon skeletons from the degration of amino acids either enter the TCA cycle or are converted to pyruvate.Glycerol, derived from the lipolysis of adipose triacylglycerol in response to low insulin when blood [glucose] is low is phosphroylated in the liver by Glycerol Kinase and reduced to dihydroxyacdetone phosphate by Glycerol 3-phosphate Dehydrogenase, which enters the gluconeogenesis pathway.
Excessive ethanol consumption limits gluconeogenesis because detoxification of ethanol competes for NAD+Click The Image
Carbon Skeletons For Gluconeogenesis
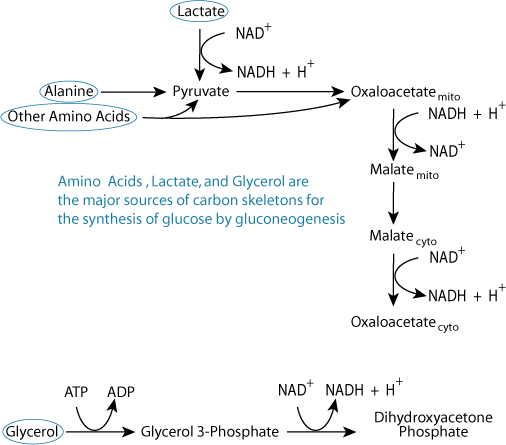 Hypoglycemia triggers the proteolysis of tissue proteins; most of the resulting amino acids are degraded in the liver to yield carbon skeletons for gluconeogenesis, either by degrading directly to pyruvate (alanine), indirectly to pyruvate (5 other amino acids), or to intermediates of the TCA cycle, which are converted to oxaloacetate in the TCA cycle. Glycerol, from the hydrolysis of adipose triacylglycerols, also induced by hypoglycemia (low [insulin]), enters the gluconeogenesis pathway at dihydroxyacetone phosphate. Lactate, produced largely in muscle and red blood cells, is converted to pyruvate by lactate dehydrogenase.
Hypoglycemia triggers the proteolysis of tissue proteins; most of the resulting amino acids are degraded in the liver to yield carbon skeletons for gluconeogenesis, either by degrading directly to pyruvate (alanine), indirectly to pyruvate (5 other amino acids), or to intermediates of the TCA cycle, which are converted to oxaloacetate in the TCA cycle. Glycerol, from the hydrolysis of adipose triacylglycerols, also induced by hypoglycemia (low [insulin]), enters the gluconeogenesis pathway at dihydroxyacetone phosphate. Lactate, produced largely in muscle and red blood cells, is converted to pyruvate by lactate dehydrogenase.Oxidized NAD Is Required
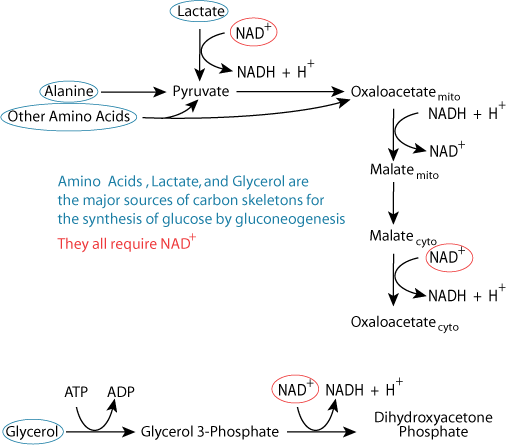 Carbon skeletons from all three major sources require reactions that use cytosolic NAD+ for conversion to gluconeogenic pathway intermediates.
Carbon skeletons from all three major sources require reactions that use cytosolic NAD+ for conversion to gluconeogenic pathway intermediates.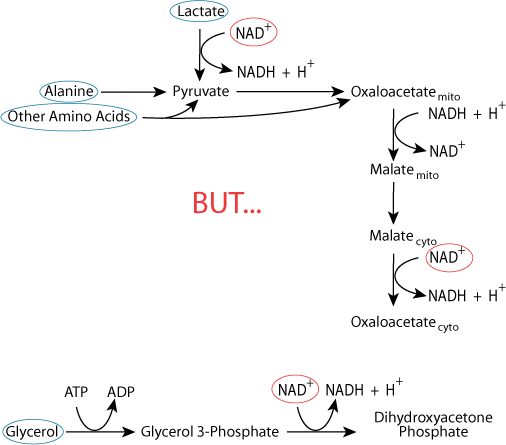 The cellular pool of NAD+ is not unlimited.
The cellular pool of NAD+ is not unlimited.Ethanol Detoxification Depletes Oxidized NAD
 Alcoholics are typically malnourished, and rely on liver glycogenalysis and gluconeogenesis to maintain blood glucose homeostasis. Liver glycogen is exhausted after about 24-48 hours, when gluconeogenesis is the only mechanism that maintains blood glucose homeostasis. However, the reactions of ethanol detoxification deplete liver NAD+, converting it to NADH faster than NADH can be reoxidized to NAD+, thereby limiting the liver's ability to synthesie glucose from non-carbohydrate carbon skeletons. Consequently, alcoholics are at risk of develooping severe hypoglycemia.
Alcoholics are typically malnourished, and rely on liver glycogenalysis and gluconeogenesis to maintain blood glucose homeostasis. Liver glycogen is exhausted after about 24-48 hours, when gluconeogenesis is the only mechanism that maintains blood glucose homeostasis. However, the reactions of ethanol detoxification deplete liver NAD+, converting it to NADH faster than NADH can be reoxidized to NAD+, thereby limiting the liver's ability to synthesie glucose from non-carbohydrate carbon skeletons. Consequently, alcoholics are at risk of develooping severe hypoglycemia.Reciprocal Regulation of Glycolysis and Gluconeogenesis: Ensuring That Both Don't Occur Simultaneously in a Futile Cycle
 Glycolysis degrades glucose for two major purposes: to generate ATP and to provide carbon skeletons for the biosynthesis of other molecules. The rate of glycolysis is regulated to fulfill both purposes.
Glycolysis degrades glucose for two major purposes: to generate ATP and to provide carbon skeletons for the biosynthesis of other molecules. The rate of glycolysis is regulated to fulfill both purposes.
Phosphofructokinase 1, which catalyzes the committed step of glycolysis, is the most important site of control. Intracellular small molecules regulate phosphofructokinase I. A high level of ATP inhibits phosphofructokinase 1 and AMP stimulates it and inhibits its fructose 1,6-bis phosphatase, opposing activity. The ATP inhibition is enhanced by citrate, which stimulates fructose 1,6-bis phosphatase, and reversed by AMP. Thus, the rate of glycolysis depends on the need for ATP, as signaled by the intracellular ATP/AMP ratio, and on the need for carbon skeleton building blocks for the synthesis of other biomolecules, as signaled by the intracellular level of citrate.
In the liver, the most important activator of phosphofructokinase 1 activity and inhibitor of fructose 1,6-bis phosphatase, its opposing activity, is fructose 2,6-bisphosphate (F 2,6-bisP), a small molecule whose level is controlled by the activity of phosphofructokinase 2, which forms it from fructose 6-phosphate, and of fructose 2,6-bisphosphatase, which degrades it to fructose 6-phosphate. The ratio of the activities of these two enzymes, which exist in a single protein molecules — the bifunctional enzyme — is regulated by protein kinase A (cAMP-dependent protein kinase), whose activity is increased in response to glucagon and decreased in response to insulin. In addition, the synthesis of fructose 1,6 bis-Phosphatase is reciprocally regulated by insulin and glucagon signaling. Thus, in the liver, the degradation (glycolysis) and production (gluconeogenesis) of glucose are reciprocally regulated by the ratio of insulin/glucagon, which is determined by the level of glucose in the blood.
The outflow of pyruvate from glycolysis and its inflow to glyconeogenesis are reciprocally regulated. Pyruvate kinase is phosphorylated, and thereby inhibited, by protein kinase A (cAMP-dependent protein kinase) in response to low blood glucose and increased when blood glucose levels rise. The activity of phosphoenolpyruvate carboxy kinase (PEPCK), in its opposing pathway, is increased as a result of increased transcription of the PEPCK gene in response to the activation of the CREB transcription factor by its phosphorylation by protein kinase A. Thus, at this point in the opposing glycolysis/gluconeogenesis pathways, regulation of the opposing enzymes prevents their simultaneous activity in a futile cycle. ADP inhibits both pyruvate carboxylase and PEPCK so that gluconeogenesis, which requires the input of ATP energy and electrons from NADH, does not proceed at the expense of the energy required to maintain hepatocyte essential functions.