PENTOSE PHOSPHATE PATHWAY
 IV. The Pentose Phosphate Shunt functions as a source of NADPH and Ribose 5-phosphate
IV. The Pentose Phosphate Shunt functions as a source of NADPH and Ribose 5-phosphate
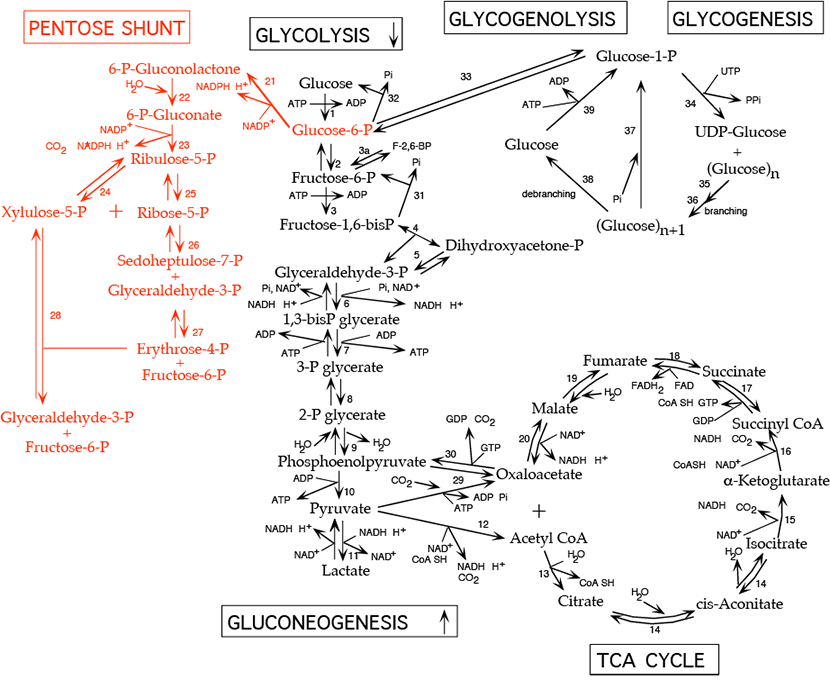
The pentose phosphate shunt (also known as the hexose monophosphate shunt or the 6-phosphogluconate pathway) shares the intermediates glyceraldehyde-3-phosphate and fructose-6-phosphate with the glycolytic pathway. Both pathways occur in the cytosol and, therefore, have access to one another. The pentose phosphate shunt has two phases, the oxidative phase (reactions 21 - 23), in which NADPH and pentose-5-phosphate are generated, and the non-oxidative phase (reactions 24 - 28), in which unused pentose-5-phosphate can be converted to other sugar intermediates and then returned to the glycolytic pathway as glyceraldehyde-3-phosphate and fructose-6-phosphate. Alternatively, pentose phosphate for nucleotide synthesis can be generated from glycolytic intermediates by the reversible reactions of the non-oxidative phase. Relative production of NADPH verses pentose-5-phosphate such as ribose-5-phosphate varies with metabolic demand. NADPH participates in biosynthetic reductions while NADH, which is not generated by the pentose phosphate shunt, is used primarily to donate electrons for oxidative phosphorylation to generate ATP. All cells require NADPH for reductive detoxification, and most cells require ribose-5-phosphate for nucleotide synthesis.
Reactions of the Oxidative Phase:
- Conversion of glucose-6-phosphate to 6-phosphogluconolactone by glucose-6-phosphate dehydrogenase:
- irreversible, rate limiting step of the pathway
- inhibited by NADPH which competes for NADP+ binding
- NADPH produced, C=O double bond formed
- Km for NAD+ is 1000-fold higher than for NADP+. In the fed state, rat liver NADP+/NADPH is about 0.14, while NAD+/NADH is about 700. This allows a high capacity for electron acceptor activity for oxidations, and at the same time, a high electron donor activity for reductive reactions, including the synthesis of fatty acids and sterols, and the reduction of inappropriately oxidized protein cysteine residues.
- X-linked gene with mosaic expression in females
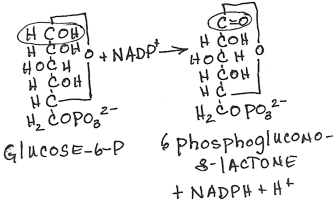
- Conversion of 6-phosphogluconolactone to 6-phosphogluconate by lactonase:
- irreversible
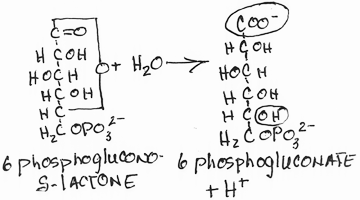
- Conversion of 6-phosphogluconate to ribulose-5-phosphate by 6-phosphogluconate dehydrogenase:
- irreversible
- NADPH produced, CO2 released
Reactions of the Non-Oxidative Phase:
- Interconversion of ribulose-5-phosphate and xylulose-5-phosphate by phosphopentose epimerase (ribulose-5-phosphate epimerase):
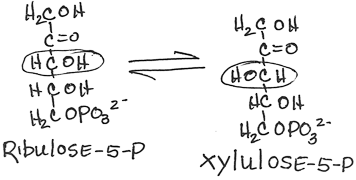
- reversible
- Interconversion of ribulose-5-phosphate and ribose-5-phosphate by phosphopentose isomerase (ribose-5-phosphate ketoisomerase):
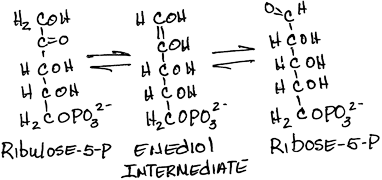
- reversible
- Ribose is a major component of nucleic acids.
- Rearrangement of (ribose-5-phosphate + xylulose-5-phosphate) to form sedoheptulose-7-phosphate and glyceraldehyde-3-phosphate by transketolase:
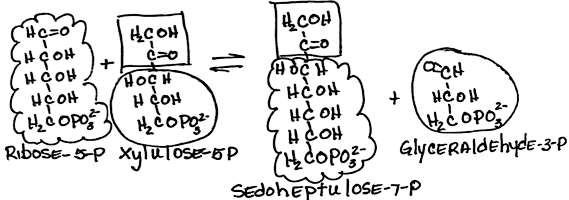
- reversible
- Glyceraldehyde-3-phosphate is a glycolysis intermediate.
- requires thiamine pyrophosphate [TPP]: Measurement of red blood cell transketolase activity is a convenient bioassay to test for TPP deficiency.
- Rearrangement of sedoheptulose-7-phosphate and glyceraldehyde-3-phosphate to form erythrose-4-phosphate and fructose-6-phosphate by transaldolase:
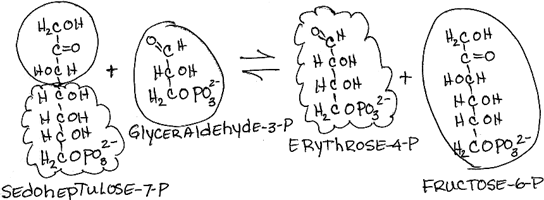
- reversible
- Fructose-6-phosphate is a glycolysis intermediate.
- Rearrangement of xylulose-5-phosphate and erythrose-4-phosphate to form glyceraldehyde-3-phosphate and fructose-6-phosphate by transketolase:
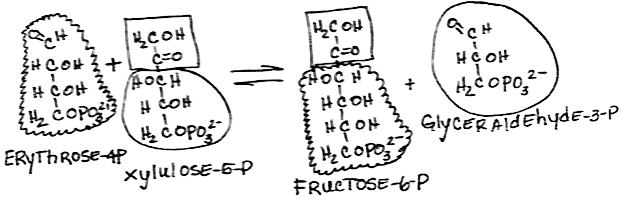
- reversible
- Both products are also glycolysis intermediates.
In Summary:
The oxidative phase of the pentose phosphate shunt can generate NADPH, without generating a net increase of ribose-phosphate by using the ribose-phosphate sugars formed as substrates for the non-oxidative phase, which converts them to fructose-6-phosphate and glyceraldehyde-3-phosphate that can then enter the glycolytic pathway.
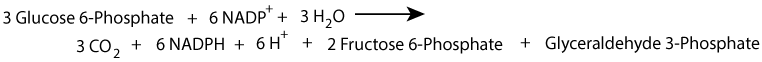
Alternatively, ribose-phosphate can be generated by the non-oxidative phase, without NADPH production, from the glycolytic intermediates fructose-6-phosphate and glyceraldehyde-3-phosphate.
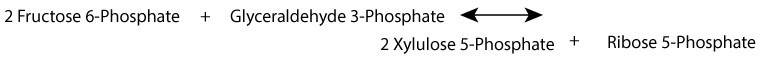
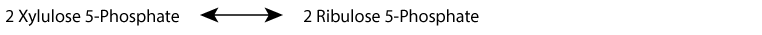
Xylulose 5-Phosphate is in equilibrium with glycolytic intermediates by the reversible reactions of the non-oxidative phase of the pentose phosphate shunt; when glucose increases, for example after a meal, xylulose 5-phosphate increases. It activates protein phosphatase 2A, which removes the regulating phosphate group from the liver bifunctional enzyme, the inactivating phosphate from pyruvate kinase, and an inactivating phosphate group from a transcription factor, ChREBP (Carbohydrate Response Element Binding Protein) that activates transcription of genes for fatty acid synthesis in the liver. Liver glycolysis increases, resulting in an increase in pyruvate and subsequently, by pyruvate dehydrogenase, an increase in acetyl CoA, the substrate for fatty acid synthesis. At the same time, transcription of the gene encoding fatty acid synthase, a multi-enzyme protein that catalyzes the synthesis of fatty acids, increases because ChREBP becomes active due to the removal of its inactivating phosphate group.
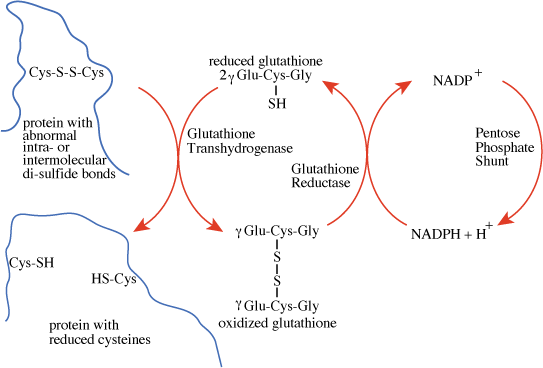 The pentose phosphate shunt maintains a reducing environment
The pentose phosphate shunt maintains a reducing environmentThe activity of the oxidative phase of the pentose phosphate shunt is low in muscle, but high in liver, testis, adrenal cortex, and mammary gland, where NADPH is used in the synthesis of fatty acids or steroids, and in red blood cells, which are exposed to strongly oxidizing conditions as a result of their oxygen carrying function and use NADPH generated by the pentose phosphate shunt to counteract their oxidizing environment.
The NADPH generated in red blood cells reduces oxidized glutathione, a tripeptide of sequence γ-glu-cys-gly, to the sulfhydryl form, catalyzed by glutathione reductase. The reduced glutathione serves as a "sulfhydryl buffer" to maintain the cysteine residues of hemoglobin and other red-cell proteins in the reduced state. It also serves to keep the hemoglobin iron in the ferrous state by reducing peroxides that undergo a reaction in which they extract an electron from ferrous iron, converting it to ferric iron, which impairs hemoglobin function.
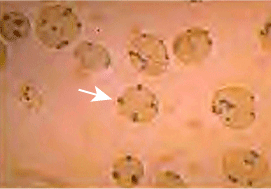 Heinz Bodies; see text
Heinz Bodies; see textAcute hemolysis from G6PD deficiency is linked to the development of Heinz bodies in red blood cells (dark brown spots — one indicated by the white arrow in the figure above), which are composed of denatured and aggregated hemoglobin.
Malaria Selects for G6PD Deficiency: G6PD deficiency is an incomplete hereditaryClick The Image
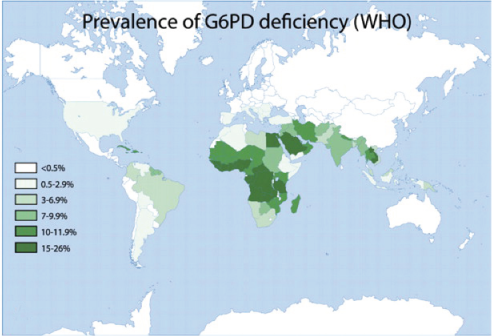 Glucose 6-phosphate dehydrogenase deficiency overlaps with malaria endemicity.
Glucose 6-phosphate dehydrogenase deficiency overlaps with malaria endemicity. 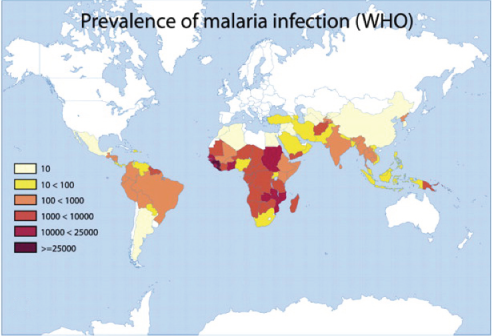 Glucose 6-phosphate dehydrogenase deficiency overlaps with malaria endemicity.
Glucose 6-phosphate dehydrogenase deficiency overlaps with malaria endemicity.Optimum Red Blood Cell (RBC) redox status is required by malaria parasites for their survival, replication, and development. Redox status is altered in G6PD deficient RBCs, supporting the idea that G6PD deficiency confers protection against malarial infection (although predisposes to hemolysis).
Favism: Fava beans contain two glycosidic compounds, vicine and convicine, which are hydrolysed enzymatically to form pyrimidine aglycones, divicine and isouramil, respectively. The proposed mechanism for the cause of favism is that these new compounds then undergo redox cycling and in the process deplete reduced glutathione, leading to the formation of free radicals and hydrogen peroxide that damage red blood cells in individuals deficient in G6PD.
For centuries, school teachers on the Mediterranean island of Sardinia have witnessed a curious phenomenon. Every February, as spring arrives and fava bean season is at its height, some of their students (mostly boys) suddenly seem drained of energy. For the next three months, their schoolwork suffers. They complain of dizziness and nausea and fall asleep at their desks. Then, just as suddenly, they return to normal and remain healthy and active until the next February rolls around. Although some merely feel a strange lethargy, others die after urinating quantities of blood. At times, as many as 35 percent of the islanders have suffered from this phenomenon.
Antimalarial drugs: It was during the Korean War that the connection was made between the Mediterranean allele of G6PD, whose gene is located on the X chromosome, and the hemolytic effects of anti-malarial drugs.
Antimalarial drugs such as pamaquin are oxidizing compounds that deplete reduced glutathione. Treatment of malaria with these drugs can cause (severe) hemolysis in G6PD-deficient individuals (i.e., males having a variant G6PD or heterozygous females with skewed
| Common Genetic Variants of Glucose-6-Phosphate Dehydrogenase (G6PD) | |||||
| Variant | Description | ||||
| B (non-mutated) | Biologic: Normal activity Clinical: No hemolysis |
||||
| A− (G202A and A376G) | Accounts for most cases of G6PD deficiency in persons
of African descent; found in 10% to 15% of black Americans and Africans from West and Central Africa
|
||||
| Mediterranean (C563T) | Most common variant in whites
| ||||
Click The Image
Reduced Glutathione
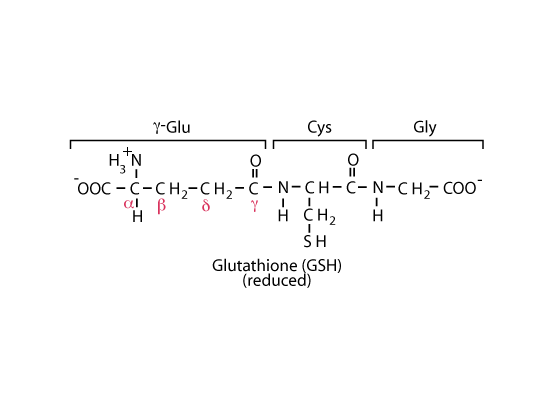
NADPH produced in the oxidative phase of the pentose phosphate shunt maintains the cysteinyl sulfur of glutathione in the reduced state.
Acetaminophen
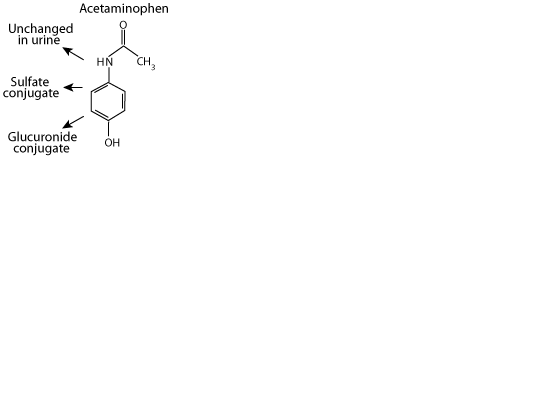
Acetaminophen, a xenbobiotic, is excreted in the urine either unchanged, as a sulfate conjugate, or as a glucuronide conjugate. However,
N-acetyl-p-benzoquinone imine (NAPQI)
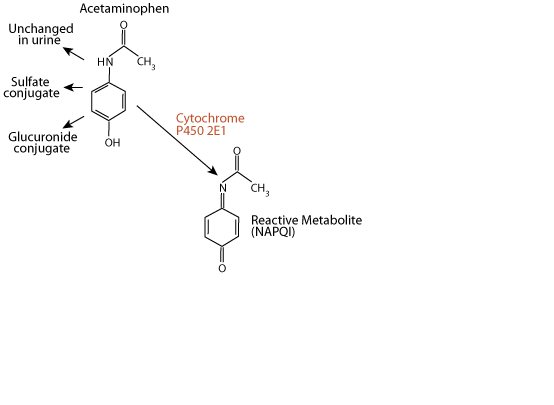
approximately 5% of a therapeutic dose is metabolized by the liver enzyme cytochrome P450 2E1 to the electrophile N-acetyl-p-benzoquinone imine (NAPQI). NAPQI is extermely toxic to the liver, as it reacts with and damages several types of functional macromolecules.
Glutathione Reacts With and Detoxifies NAPQI
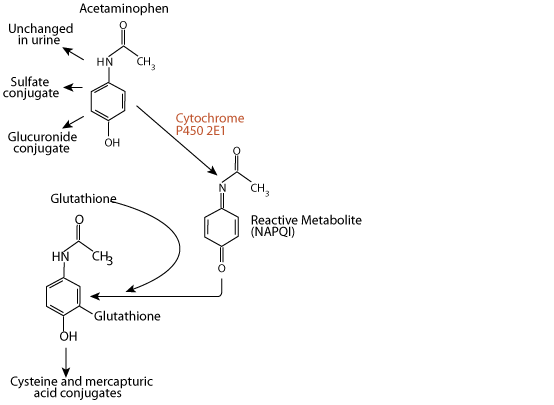
Ordinarily, NAPQI is rapidly detoxified by interaction with glutathione to form cysteine and mercapturic acid conjugates.
Overdoses of Acetaminophen Deplete Liver Glutathione
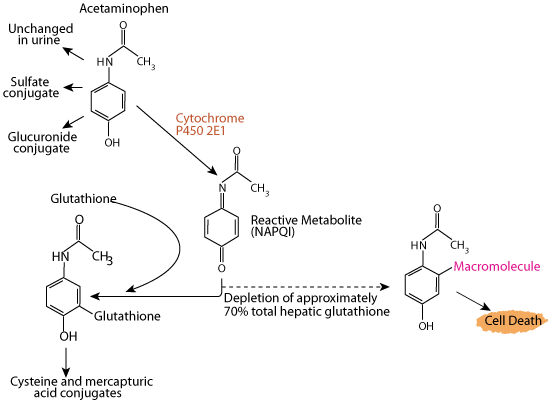
Acetaminophen (e.g. Tylenol) is a commonly used analgesic and antipyretic agent, and its use is one of the most common causes of poisoning worldwide. If glutathione is depleted by an excessive intake of acetimenophen, NAPQI accumulates and interacts with various macromolecules, leading to hepatocyte injury and potentially death from fulminant liver failure. Acetaminophen poisoning can be due to ingestion of a single overdose (usually as an attempt at self-harm) or ingestion of excessive repeated doses or too-frequent doses, with therapeutic intent. Data compiled by the U.S. Food and Drug Administration linked as many as 980 deaths in a year to overdoses of drugs containing acetaminophen that cause depletion of glutathione resulting in dammage to the liver.
Warning On Extra Strength Tylenol Package Insert

Johnson & Johnson incurred lawsuits because of cases of liver failure resulting from the use of Tylenol. Packages of Extra Strength Tylenol now contain the warning: “Acetaminophen overdose is one of the most common poisonings worldwide” — according to the National Institutes of Health.
The cysteinyl sulfur of glutathione (GSH) is maintained in the reduced state by NADPH produced in the oxidative phase of the pentose phosphate shunt and provides a nucleophilic thiol important for the detoxification of electrophilic metabolites and metabolically-produced oxidizing agents. The net negative charge and overall hydrophilicity of GSH greatly increases the aqueous solubility of the lipophilic moieties with which it becomes conjugated. Its unique tripeptide structure, including the N-terminal glutamyl residue linked via a γ-glutamyl peptide bond, provides for specificity in GSH transferase enzymes.
In mammals, GSH conjugates are often further metabolized by hydrolysis and N-acetylation, either in the gut or in the kidney, to give N-acetylcysteinyl conjugates known as mercapturic acids, which are excreted in the urine. Numerous xenobiotics have been shown to be excreted as mercapturic acids, and it has been suggested that enzymes, the GSH transferases, catalyze the initial conjugation with GSH. Conjugation can be either enzymic or non-enzymatic, nevertheless, GSH transferases are widespread in both animals and plants, and enzymatic conjugation is assumed to be particularly effective and may even result in GSH depletion.
All tissues appear to possess some GSH transferase enzymatic activity, and most have more than one enzyme species - usually a spectrum of isoenzymes that is characteristic of the tissue. The multiplicity of GSH transferases is probably due to the need to deal with a large range of electrophiles. However, it is not clear what the electrophiles encountered by various tissues are, although it is assumed that in the liver, which is so important in detoxication, most of the electrophiles are derived from xenobiotics.