PROTEIN SECONDARY STRUCTURE
ALPHA-HELICES & BETA-PLEATED SHEETS
Four terms are useful to distinguish features of the structures of proteins:
- Primary structure: the structure of covalent bonds holding the atoms of amino acids together and attaching individual amino acids to one another; the amino acid sequence of a protein.
- Secondary structure: regular structural motifs formed by hydrogen bonding among atoms of the peptide backbone (i.e., motifs not directly involving the atoms of the amino acid side chains).
- Tertiary structure: the complete three-dimensional conformation of a polypeptide chain.
- Quaternary structure: the complete three-dimensional structure of a complex of two or more polypeptide chains.
Here, protein secondary structure is reviewed. Two structural motifs, alpha-helices and beta-pleated sheets, are crucially important: they are particularly common and provide scaffolds on which other features of many proteins are organized. General structural features of polypeptide chains were deduced from X-ray crystallographic studies of small peptides by Linus Pauling and his colleagues, who then asked whether long range polypeptide conformations could be found consistent with these features. (Pauling’s goal was to identify common structural features of whole proteins, but the techniques needed to interpret X-ray diffraction data for such large molecules had not yet been invented. A critical intellectual advance was thus his realization that the structures of small molecules like dipeptides, which could be worked out in atomic detail with the available technology, could be used as the basis for model building studies to deduce what a whole protein might look like. This same process of model building and extrapolation was used by Watson and Crick a few years later to deduce the double-helical structure of DNA from the DNA fiber-diffraction patterns that Rosalind Franklin had obtained.)

First, the peptide bond is flat. Electron delocalization yields a long C-O distance compared to the one expected for a carbon-oxygen double bond, a short C-N distance compared to the one expected for a carbon-nitrogen single bond, and forces all the affected atoms to lie in one plane. The peptide bond can be seen as a plate, containing elements of two adjacent amino acid residues. The alpha-carbon forms a ball joint about which adjoining rigid plates rotate as indicated by the arrows in the diagram.
Second, extensive hydrogen bond formation stabilizes polypeptide conformations. (Continuing the history lesson, Pauling was among the first to characterize hydrogen bonds and to recognize that these weak noncovalent interactions could play a major role in determining the conformations of organic molecules. He was eager to find new uses for his favorite bond type.) The hydrogen bonds of interest here form between the amide hydrogens and carbonyl oxygens of the peptide backbone. The hydrogen-bonding possibilities of the various side chains are not considered.
Two regular conformations of polypeptide chains, alpha-helices and beta-pleated sheets, are consistent with the planar structure of the peptide bond, allow covalent bonds to be at angles consistent with their potential energy minima; and allow maximal linear H-bond formation. Other stable, regular conformations are also possible. These require relaxing one or more of the optimization requirements listed here and we do not need to enumerate them.
The alpha-helix. A single continuous stretch of amino acid residues is organized into a compact columnar structure. The leftmost panel of the diagram shows a simplified view, with each amino acid residue represented only by its alpha-carbon (gray ball) and its side chain (green ball). There are about 3.6 amino acid residues per turn of the helix.

The side chains of the amino acid residues protrude away from the helix. They play no direct role in forming the helical structure but in actual proteins play a critical role in determining whether any given stretch of polypeptide is likely to take on a stable helical conformation. As shown in the top diagram on the left, in which the helix is rotated 90 out of the plane of the picture, to view it from the top, nearby side chains are in close proximity. If two such chains are both bulky, for example, steric hindrance will occur. If they have like charges repulsion will occur, and in such cases formation of a helix will not be favored. Conversely, side chains with opposite charges, or ones that can interact by other means such as Van de Waals interactions, may favor the formation of a particular stretch of a protein into a helical conformation.
A final important structural feature of the alpha helix is shown in the bottom left part of the diagram. Here, the atoms in the helix are drawn with accurate Van de Waals radii, and the diagram shows that the helix is a solid column – there is no accessible empty space in its center.
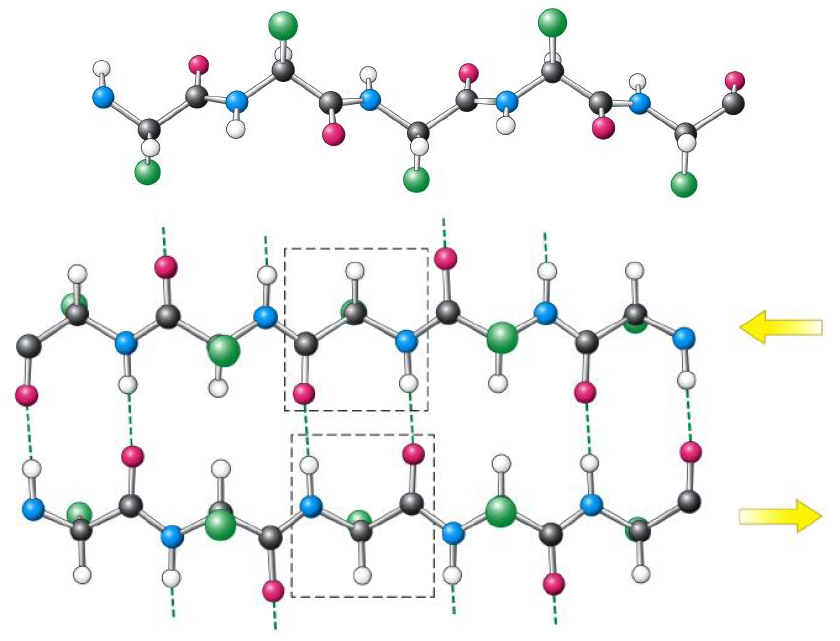
If successive strands have the same amino-to-carboxy sense, a parallel beta-pleated sheet forms. Its hydrogen bonds are somewhat distorted; this conformation is less energetically favorable but can still be stable and is observed in proteins.
If an alpha-helix has the architectural role in a protein of a column, the beta-pleated sheet has the role of a rigid plate or slab.