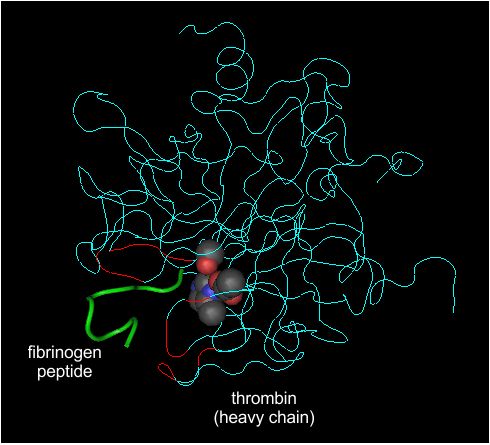
Left panel Peptide backbone of thrombin (blue and red) interacting with part of fibrinogen (green).
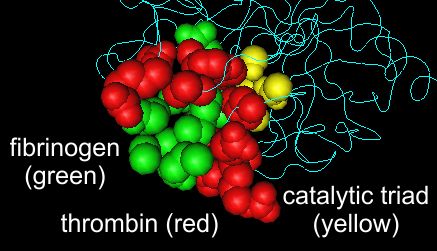
Right panel Same structure as in the left panel, but showing a space filling model of thrombin's recognition site (red) and catalytic triad (yellow).
Like all serine proteases, thrombin has both a catalytic site and a substrate recognition site.
The peptide to be cleaved is held in the recognition site so that the target peptide bond is right next to the serine of the catalytic triad.
The recognition site of thrombin consists of two flaps that surround the target peptide.
The target peptide is held in place by a combination of charged and hydrophobic interactions.
Interactions that are responsible for thrombin's specificity.
The thrombin amino acids that interact directly with fibrinogen are shown in red. This structure can be accessed via the NCBI structure database as structure 1FPH.

|

Left panel Peptide backbone of thrombin (blue and red) interacting with part of fibrinogen (green). Right panel Same structure as in the left panel, but showing a space filling model of thrombin's recognition site (red) and catalytic triad (yellow). |