BASIC FEATURES OF POLYPEPTIDE CHAINS
Two amino acids react with the elimination of a water molecule to form a dipeptide. Additional amino acids are added to form a polymeric structure, a polypeptide.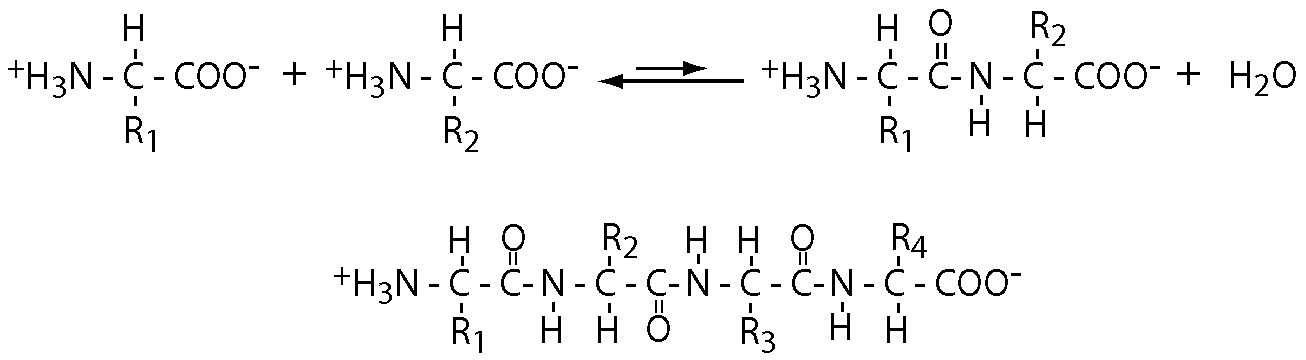
As indicated in the diagram, this reaction is unfavorable. Hydrolysis is favored but the activation energy needed to initiate hydrolysis is large so peptides and proteins can be quite stable under physiological conditions.
The bottom part of the diagram illustrates several key features of polypeptides.
- They are linear. While the amino groups in lysine side chains and the carboxyl groups in aspartate and glutamate side chains are chemically capable of forming peptide bonds, they do not do so in proteins.
- A polypeptide chain thus has a single amino terminal end and a single carboxyl terminal end. Polypeptides are represented in an amino-to-carboxy direction.
- Both the composition and the precise sequence of a polypeptide chain are important in determining its function: +H3N-alanine-leucine-COO- has distinct chemical properties from +H3N-leucine-alanine-COO- . The number of possible distinct polypeptides is thus enormous.
What is the difference between a polypeptide and a protein? For our purposes, it is the outlook of the person using the word: a chemist interested in the structural features of the molecule might naturally call it a polypeptide while a biologist interested in its role in a cell might naturally call it a protein.