PROPERTIES OF AMINO ACIDS
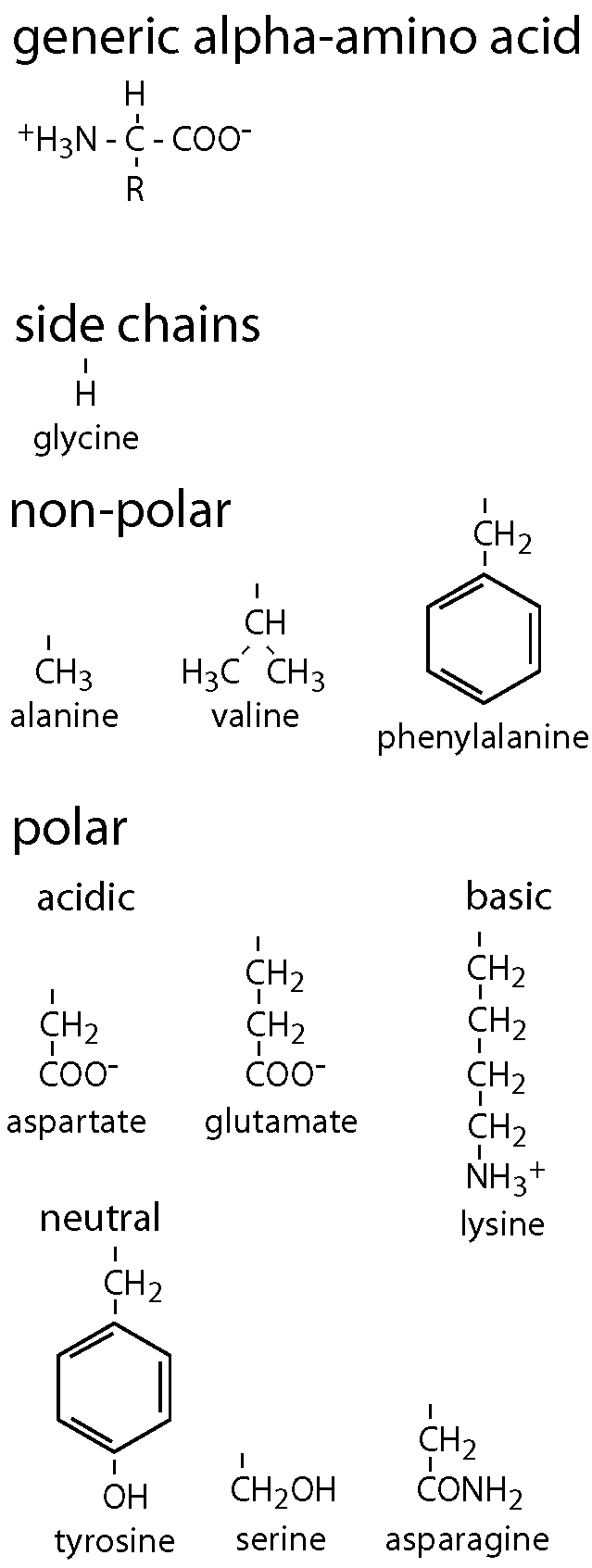
Twenty different amino acids can be incorporated into proteins synthesized in the human body. All are alpha-amino acids, that is, their amino groups are attached to the carbon adjacent to their carboxyl groups, as shown in the diagram of a generic amino acid. The carboxyl group is a weak acid, with a pK of approximately 2, so it typically is dissociated (COO-) at physiological pH. The amino group is a weak base, with a pK of approximately 9, so it typically is fully protonated (NH3+) at physiological pH. An amino acid is thus a zwitterion and while it has no net charge, it is strongly polar and water-soluble.
Another property of (almost) all amino acids is clear from the diagram: unless the “R” group attached to the alpha carbon is a hydrogen atom (as in the amino acid glycine), the alpha carbon has four different substituents and is therefore asymmetric. All the amino acids except glycine found in human proteins are L-stereoisomers.
The twenty amino acids are distinguished from one another by their “R” groups (side chains) and it is helpful to divide them into two classes, ones with non-polar (hydrophobic) side chains and ones with polar (hydrophilic) ones. The non-polar class includes alanine, valine, and phenylalanine, shown in the diagram, as well as leucine, isoleucine, tryptophan, methionine, and proline. The polar class is divided into side chains that are negatively charged at neutral pH (acidic, aspartate and glutamate), positively charged at neutral pH (basic, lysine, as well as arginine and histidine), and uncharged but polar (tyrosine, serine, asparagine, as well as threonine, glutamine, and cysteine).
Memorizing these twenty structures is not necessary, but being able to recognize them and understand how their different side chains determine their distinctive properties both as free molecules and as components of proteins is essential.