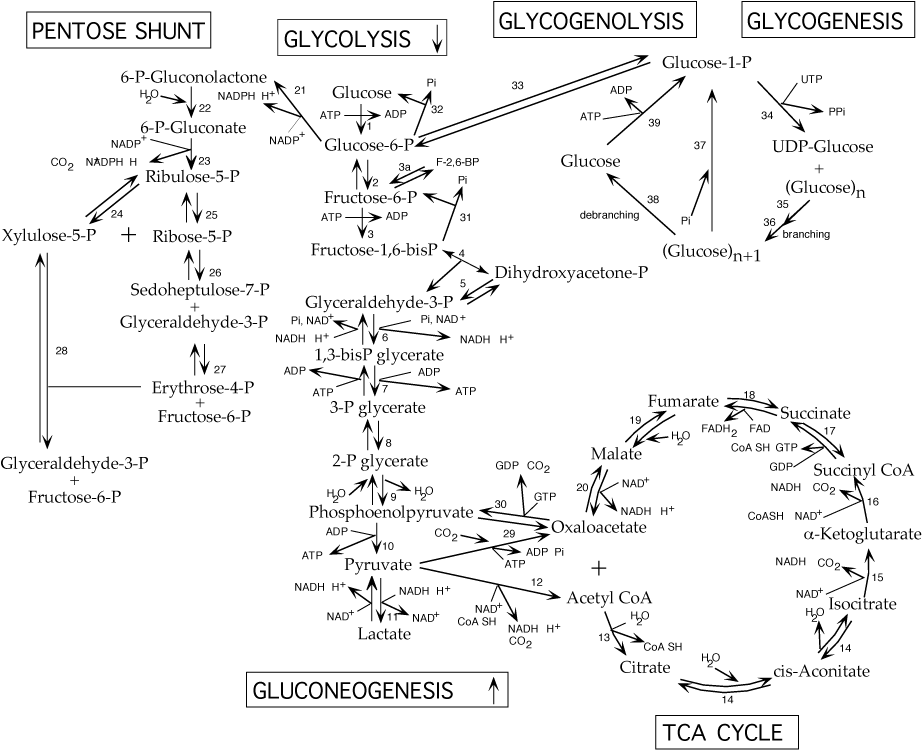
REVERSIBLE & REGULATED STEPS OF GLUCOSE METABOLISM
Trapping Glucose In Cells And Release Of Glucose To The Blood
Phosphorylation of Glucose to yield Glucose-6-phosphateThe first step in glucose utilization is its phosphorylation, which prevents its transport out of cells due to the negative charge conferred by the phosphate group.
1. Hexokinase:
irreversible; allosterically inhibited by its product, glucose 6-phosphate; Km = 0.1 mMGlucokinase:
present in liver and pancreatic beta cells; irreversible; NOT inhibited by its product, glucose 6-phosphate; Km = 10 mM; when blood glucose levels are low the enzyme level is reduced (transcription of the gene is reduced and the glucokinase protein is sequestered by the Glucokinase Regulatory Protein), thereby reducing the retention of glucose produced by glycogenolysis and gluconeogenesis in liver cellsDephosphorylation of Glucose-6-phosphate to yield Glucose
The dephosphorylation of glucose allows it to be transported out of cells
32. Glucose-6-phosphatase:
present in liver and kidney; located in the endoplasmic reticulum; irreversible; allows liver and kidney to supply glucose, recovered from stored glycogen or synthesized from 3-carbon precursors by gluconeogenesis, to other tissues during fasting; liver is the major tissue that supplies glucose to other tissues; during severe fasting the kidney also supplies an appreciable amount of glucose. Glucose-6-phosphatase gene transcription is increased by phosphorylated CREB when blood glucose is low (signaled by glucagon)
Glycolysis and Gluconeogenesis
Phosphorylation of Fructose-6-phosphate to yield Fructose-1,6-bisphosphateThis is the committed step of glycolysis, and is highly regulated.
3. Phosphofructokinase 1:
irreversible; allosterically regulated: ATP increases the Km for fructose 6-phosphate thereby slowing enzymatic activity and the rate of glycolysis; AMP reverses the inhibitory effect of ATP; inhibited by low pH; inhibited by high citrate concentration which enhances the inhibitory effect of ATP; stimulated by fructose-2,6-bisphosphate which lowers the Km for fructose-6-phosphate and decreases inhibition by ATP thereby increasing the rate of glycolysisDephosphorylation of Fructose-1,6-bisphosphate to yield Fructose-6-phosphate
Note that the dephosphorylation of fructose-1,6-bisphosphate and the phosphorylation of fructose-6-phosphate are reciprocally regulated by AMP, fructose-2,6-bisphosphate and citrate. This prevents glycolysis and gluconeogenesis from occurring simultaneously.
31. Fructose-1,6-bisphosphatase:
irreversible; allosterically regulated: inhibited by AMP; inhibited by fructose-2,6-bisphosphate; inhibited by fructose-6-phosphate; stimulated by citrate. Its synthesis is reciprocally regulated by insulin and glucagon.Phosphorylation of Fructose-6-phosphate to yield Fructose-2,6-bisphosphate and dephosphorylation of Fructose-2,6-bisphosphate to yield Fructose-6-phosphate by two different activities of a single protein, a bifunctional enzyme
Fructose-2,6-bisphosphate is synthesized by phosphofructokinase 2 in small amounts and acts as a "feed-forward" activator of phosphofructokinase 1. Fructose-6-phosphate stimulates the synthesis of fructose-2,6-bisphosphate, which then stimulates phosphofructokinase 1 to synthesize fructose-1,6-bisphosphate. The bifunctional enzyme is a single protein that has both phosphofructokinase 2 and fructose-2,6-bisphosphatase activities. Its activity is regulated by protein phosphorylation/dephosphorylation. Note below the reciprocal regulation of the two opposing enzymatic activities of this single protein.
3a. Phosphofructokinase 2:
stimulated by fructose-6-phosphate; Cyclic AMP-dependent protein kinase (protein kinase A) phosphorylates and inhibits the liver enzyme in response to either glucagon, which signals low blood glucose levels, or epinephrine; heart muscle isoform is stimulated by phosphorylation in response to epinephrine, skeletal muscle isoform is not phosphorylated.
3a. Fructose-2,6-bisphosphatase:
inhibited by fructose-6-phosphate; Cyclic AMP-dependent protein kinase (protein kinase A) phosphorylates and stimulates the liver enzyme in response to either glucagon, which signals low blood glucose levels, or epinephrine.Production of Pyruvate from Phosphoenolpyruvate
This is the last step of glycolysis and is highly regulated to control the outflow of glycolytic products.
10. Pyruvate Kinase:
Production of Phosphoenolpyruvate from Pyruvateirreversible; ATP produced; allosterically regulated: inhibited by ATP; inhibited by alanine; "feed forward" stimulated by fructose-1,6-bisphosphate which signals a high level of glycolytic products are available; regulated by phosphorylation: cyclic AMP-dependent protein kinase (protein kinase A) phosphorylates and inactivates the liver (but not the muscle) enzyme in response to low blood glucose signalled by glucagon
This is a two step reaction in which oxaloacetate is first produced from pyruvate and then phosphoenolpyruvate is produced from oxaloacetate. The two steps expend more energy than is harvested when pyruvate is produced from phosphoenolpyruvate.
29. Pyruvate carboxylase:
irreversible; oxaloacetate produced; ATP expended; located in mitochondria; allosterically regulated: activated by acetyl CoA, inhibited by ADP; the oxaloacetate produced is reduced (at the expense of NADH + H+) to malate for transport from the mitochondria to the cytoplasm, where it is re-oxidized to oxaloacetate (at the expense of NAD+); Note that the stimulation of oxaloacetate production during acetyl CoA excess could help speed up the TCA cycle by providing sufficient oxaloacetate to combine with the excess acetyl CoA, but if a surplus of ATP is available the oxaloacetate is directed towards gluconeogenesis.
30. Phosphoenolpyruvate carboxykinase:
inhibited by ADP; GTP expended: the phosphate of GTP is transferred to oxaloacetate and CO2 is lost to generate phosphoenolpyruvate; transcription of the gene is regulated by the activity of cyclic AMP-dependent protein kinase (protein kinase A), and is responsive to blood glucose levels via the glucagon and insulin signaling pathways.
| RECIPROCAL REGULATION OF GLYCOLYSIS AND GLUCONEOGENESIS | |||||||
| GLYCOLYSIS | GLUCONEOGENESIS | ||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
There are no opposing enzymatic steps. Acetyl CoA cannot be used to generate pyruvate. | ||||||
Acetyl CoA
Formation of Acetyl CoA from PyruvateAcetyl CoA is the link between glycolysis and the TCA cycle. Pyruvate, a 3-carbon structure, is converted to acetyl CoA with the loss of CO2. The 2-carbon acetate subsequently enters the TCA cycle where it is completely oxidized to 2 CO2.
12. Pyruvate~dehydrogenase:
irreversible; occurs in mitochondria; inhibited competitively by acetyl CoA (note that acetyl CoA stimulates pyruvate carboxylase which directs pyruvate towards gluconeogenesis) and by NADH which are products of the reaction; regulated by a protein kinase that is NOT the cyclic AMP-dependent protein kinase: phosphorylation of pyruvate dehydrogenase, which is stimulated by high NADH/NAD+, acetyl CoA/CoA or ATP/ADP inhibits enzymatic activity; pyruvate activates by inhibiting the protein kinase; insulin stimulates by activating a phosphatase that removes the protein phosphate. Ca2+ released from the sarcoplasmic reticulum of heart muscle activates the phosphatase to increase energy for increased heart beat.
The TCA Cycle
The final step in the oxidation of fuel molecules. Acetate generated from glucose (and other fuel molecules) as acetyl CoA is ultimately oxidized to 2 CO2 with the generation of 1 GTP and 4 reducing equivalents (3 NADH and 1 FADH2 ) from each acetate molecule that enters the cycle. These reducing equivalents are used to generate ATP via oxidative phosphorylation, where oxygen is the ultimate electron acceptor.
Formation of alpha-Ketoglutarate from IsocitrateThe first of four oxidation reactions of the TCA cycle and the first decarboxylation
15. Isocitrate dehydrogenase:
irreversible; NADH and CO2 produced (oxidative decarboxylation); actually a two step reaction in which oxidation of isocitrate to oxalosuccinate occurs first, followed by decarboxylation; ADP enhances the cooperative binding of NAD+ and isocitrate to the enzyme; ATP inhibits; NADH inhibits by displacing NAD+ from the enzyme. Ca2+ released from the sarcoplasmic reticulum of skeletal muscle speeds up the enzyme to supply increased energy for muscle contraction.Formation of Succinyl CoA from alpha-Ketoglutarate and CoA
The second of four oxidation reactions of the TCA cycle and the second decarboxylation
16. alpha-ketoglutarate~dehydrogenase:
irreversible; NADH and CO2 produced (oxidative decarboxylation); inhibited by NADH and succinyl CoA; the oxidation allows the formation of the energy-rich thioester bond. Ca2+ released from the sarcoplasmic reticulum of skeletal muscle speeds up the enzyme to supply increased energy for muscle contraction.
Glycogenesis and Glycogenolysis
Glycogenesis and glycogenolysis are reciprocally regulated.The two opposing, regulated enzymes are glycogen synthase (glycogenesis) and glycogen phosphorylase (glycogenolysis). Each is regulated by phosphorylation/dephosphorylation and by binding of other molecules. Phosphorylation of glycogen synthase switches it to the inactive, or "b " form. Phosphorylation of glycogen phosphorylase switches it to the active, or "a " form. The "b " forms of each enzyme can become active by binding small molecules. The differential response of the liver and muscle isoforms to different small molecules (glucose, AMP, ATP -see below) is in keeping with the different functions of liver and muscle in glucose metabolism. Muscle uses glucose as a primary fuel, on site, while the liver does not use glucose as a primary fuel, but rather acts to maintain blood glucose homeostasis. Glycogen synthase is phosphorylated directly by cyclic AMP-dependent protein kinase (protein kinase A) in response to glucagon or epinephrine. Glycogen phosphorylase is phosphorylated by phosphorylase kinase, which becomes active when phosphorylated by cyclic AMP-dependent protein kinase (protein kinase A). Protein phosphatase 1, which removes protein phosphates, reverses the effect of phosphorylation. Protein phosphatase 1 is regulated by two mechanisms: (1) when phosphorylated by cyclic AMP-dependent protein kinase (protein kinase A), its G (for glycogen binding) subunit is prevented from binding to glycogen particles, (2) when phosphorylated by cyclic AMP-dependent protein kinase (protein kinase A), inhibitor 1, a small protein, inhibits protein phosphatase 1. Insulin activates protein phosphatase 1. .sp A reciprocal relationship, effected by protein phosphatase 1, exists between the activities of glycogen phosphorylase (glycogen breakdown) and glycogen synthase (glycogen synthesis). Protein phosphatase 1 binds phosphorylase " a " when glucose is low, but does not dephosphorylate phosphorylase a. When glucose levels rise, glucose binds to phosphorylase a and causes a conformational change in its structure. This allows protein phosphatase 1 to dephosphorylate phosphorylase a, converting it to phosphorylase b, which is unable to bind protein phosphatase 1. Protein phosphatase 1 is thereby freed to dephosphorylate, and activate, glycogen synthase.
35. Glycogen synthase:
cyclic AMP-dependent protein kinase (protein kinase A) phosphorylates and inactivates (switches it to the "b " form), high level of glucose-6-phosphate overcomes the inhibition; insulin activates by activating protein phosphatase 1, which removes inhibiting phosphates from the synthase, thereby switching it to the active, or "b " form; glucose activates by causing the release of protein phosphatase 1 from phosphorylase so it becomes free to dephosphorylate glycogen synthase.
37. Glycogen phosphorylase:
cyclic AMP-dependent protein kinase phosphorylates phosphorylase kinase, thereby activating it to phosphorylate glycogen phosphorylase, which is thereby activated (phosphorylase a); protein phosphatase 1 dephosphorylates thereby inactivating it (phosphorylase b); the muscle "b " isoform is activated by AMP, ATP reverses the effect of AMP by competing with it for binding to the enzyme; the muscle "b " isoform is inactivated by glucose-6-phosphate; AMP has no effect on the liver isoform of phosphorylase; the liver isoform of phosphorylase is deactivated by the binding of glucose, which causes a conformational change and exposes the protein phosphate to the dephosphorylating activity of protein phosphatase 1.