TCA CYCLE
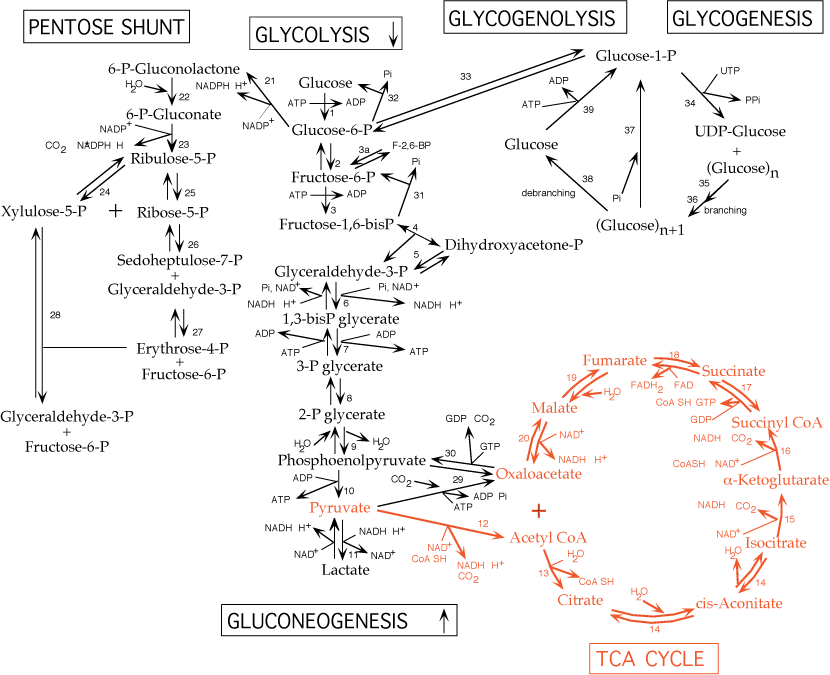 III. The Tricarboxylic Acid [TCA] Cycle occurs in mitochondria.
III. The Tricarboxylic Acid [TCA] Cycle occurs in mitochondria.
Each turn of the cycle produces one high-energy phosphate bond in the formation of GTP (high energy phosphate transferable to ADP to form ATP) and 4 reducing equivalents (3 NADH and 1 FADH2). After the O2-dependent processes of electron transport and oxidative phosphorylation, the total number of ATP produced per cycle is 10 (2.5 ATP from each NADH oxidized, 1.5 ATP from oxidation of FADH2 and 1 GTP + ADP <—> GDP + ATP ) Two molecules of pyruvate are generated from one molecule of glucose, fueling two turns of the TCA cycle. Glycolysis of 1 molecule of glucose (6 carbons) yields a net of two molecules of ATP, 2 molecules of NADH (= 5 ATP) and two molecules of pyruvate (3 carbons). Each of the two molecules of pyruvate generated per glucose molecule is converted to acetyl CoA (step # 12), producing one molecule of NADH (= 2 NADH, or 5 ATP per molecule of glucose). Acetyl CoA then enters the TCA cycle. Thus the complete oxidation of one molecule of glucose yields either 30 or 32 ATP, depending on which of two biochemical shuttles carries the electrons from the NADH produced by glycolysis across the mitochondrial membrane to the electron transport chain. Anaerobic glycolysis of glucose to lactate nets only 2 ATP per glucose molecule — no electrons from glucose are passed to the electron transport chain and no ATP is generated by oxidative phosphorylation.
- Formation of acetyl CoA from pyruvate by pyruvate dehydrogenase:
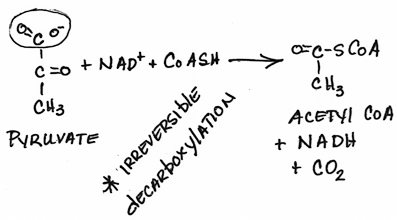
- pyruvate moves between cytosolic and mitochondrial compartments by carrier-mediated transport.
- irreversible
- NADH produced, CO2 released
- deactivated by phosphorylation by a protein kinase activity stimulated by high NADH/NAD+, acetyl CoA/CoA or ATP/ADP ratios
- pyruvate and ADP activate by inhibiting the kinase
- dephosphorylation (by a protein phosphatase), and consequently enzymatic activity, is stimulated by insulin.
- Ca2+ activates the phosphatase in heart muscle to increase energy for rapid contraction
- acetyl CoA, NADH are competitive inhibitors
- 4 vitamins are required as cofactors: thiamin (as thiamine pyrophosphate, TPP), riboflavin (as FAD), niacin (as NAD+) and pantothenate (as Coenzyme A). Lipoate, non-essential in the diet, is also required.
Alcoholism, in which ethanol consumption interferes with thiamine absorption, combined with poor nutrition, can deplete thiamin, thereby blocking the TCA cycle. For such malnourished individuals, glucose administration without thiamin supplementation is harmful, because the aerobic metabolism of glucose is compromised and lactate (ionized lactic acid) and its dissociated H+ accumulate, potentially resulting in lactic acidosis.
Click The Image
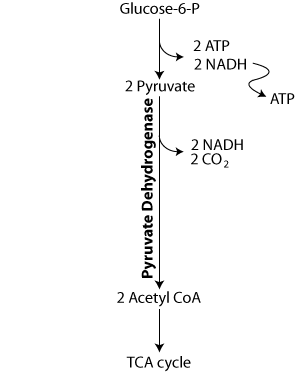
Pyruvate is oxidized by pyruvate dehydrogenase, which requires 4 vitamins, including thiamine, to yield acetyl CoA, which enters the TCA cycle.
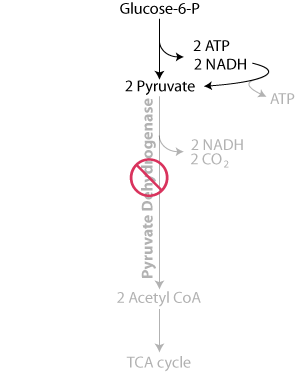
In thiamine deficiency, pyruvate dehydrogenase activity is reduced, slowing the oxidation of pyruvate to acetyl CoA, thereby limiting energy production in the mitochondria.
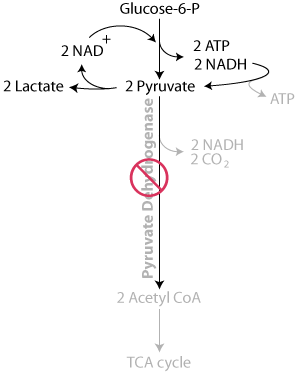
Consequently, the concentration of pyruvate increases and the electrons collected as NADH are used to reduce the pyruvate to lactate by lactate dehydrogenase, similarly as in oxygen deficiency (anaerobic conditions), instead of being transferred to the mitochondrial electron transport chain, where they would have been used in the generation of ATP.
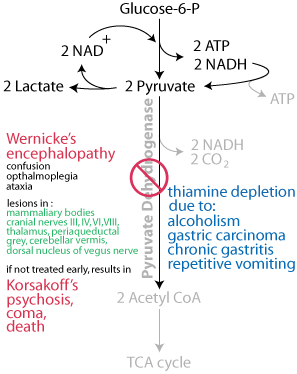
Wernicke's encephalopathy begins abruptly, presenting with the classic triad of encephalopathy, opthalmoplegia and ataxia. Untreated, it may progress to Korsakoff's psychosis, coma and death. Either an energy deficit or the build up of lactate — ionized lactic acid, which lowers the pH — or both induce the symptoms of Wernicke's encephalopathy.
Question:
An ambulance transports a comatose, disheveled patient who was found on the street and has an oder of alcohol on his breath to the emergency room. A blood analysis indicates a [glucose] of 27 mg/dL (normal: 64.8 - 104.4 mg/dL). The patient is given intravenous glucose and the patient regains responsiveness, but several hours later shows signs of acidosis and Wernicke's encephalopathy. How should this patient have been treated?It is well known that chronic alcoholics are at high risk for being deficient in vitamin B1 (thiamine), which is known to put the patient at an increased risk for Wernicke-Korsakoff Syndrome, cerebellar degeneration, and cardiovascular dysfunction. The current standard of treatment for such patients is to give them thiamine 100 mg intravenously before administering glucose containing IV fluids and then to continue this dose for several days.
- Condensation of oxaloacetate and acetyl CoA by citrate synthase to form citrate:
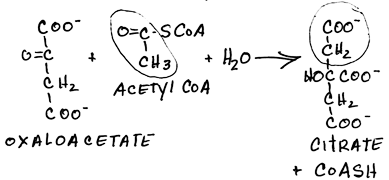
- irreversible
- feed-back inhibited by citrate (the product of the enzymatic reaction)
- ordered binding of substrates: oxaloacetate binding changes the enzyme conformation to accept acetyl CoA
- Remember, citrate is an allosteric inhibitor of reaction #3, the rate-limiting step of glycolysis catalyzed by PFKI.
- Citrate can exit the mitochondria to the cytosol, where it is used for fatty acid synthesis (discussed in the lectures on the metabolism of triacylglycerol).
- Interconversion of citrate, cis-aconitate and isocitrate by aconitase:
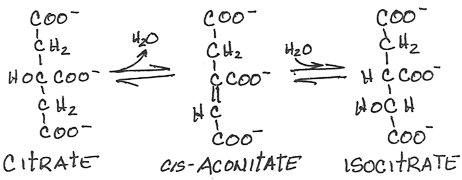
- reversible
- Conversion of isocitrate to alpha-ketoglutarate by isocitrate dehydrogenase:
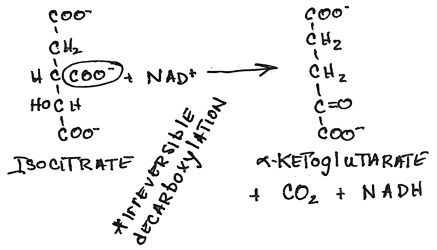
- irreversible oxidative decarboxylation
- NADH produced, C02 released
- major regulation step of the cycle responsive to the energy charge of the cell
- the cooperative binding of isocitrate and NAD+ is enhanced by ADP (reduced energy)
- competitively inhibited by NADH (increased reducing power for ATP generation)
- Ca2+, released from the sarcoplasmic reticulum in contracting heart muscle, and possibly other muscle as well, lowers the Km and increases the velocity of the enzyme to increase energy for contraction
- Conversion of alpha-ketoglutarate to succinyl CoA by alpha-ketoglutarate dehydrogenase:
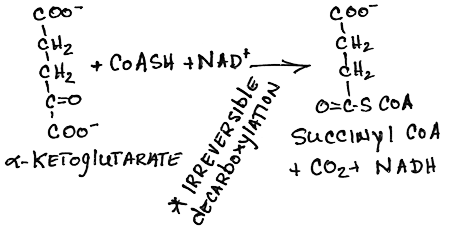
- irreversible oxidative decarboxylation
- NADH produced, CO2 released
- product inhibited by succinyl CoA and NADH
- Ca2+, released from sarcoplasmic reticulum in contracting heart muscle, and possibly other muscle as well, lowers the Km and increases the velocity of the enzyme to increase energy for contraction
- Formation of succinate from succinyl CoA by succinyl CoA synthetase (succinate thiokinase):
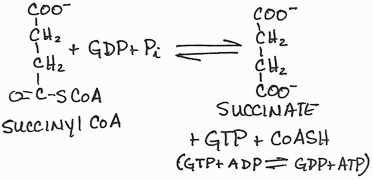
- substrate-level phosphorylation; GTP (= ATP) produced
- succinyl CoA reacts with glycine in the mitochondria to form the first intermediate, δ-aminolevulinic acid, which exits the mitochondria to the cytosol, in the heme synthetic pathway
- Conversion of succinate to fumarate by succinate dehydrogenase:
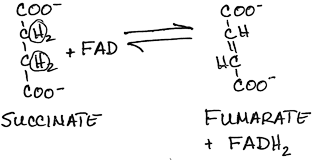
- FADH2 produced, a C=C double bond is formed
- Succinate dehydrogenase is a membrane-bound protein located on the inner mitochondrial membrane, unlike the other enzymes of the TCA cycle, which are located in the mitochondrial matrix.
- Conversion of fumarate to malate by fumarase:
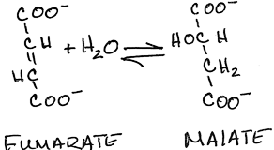
- reversible
- hydration of the fumarate double bond
- Oxidation of malate to form oxaloacetate by malate dehydrogenase:
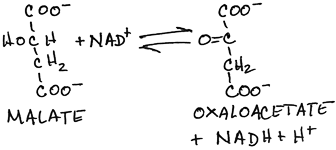
- NADH produced
Summary of the TCA cycle:
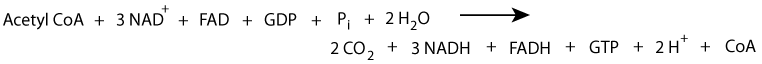
Click The Image
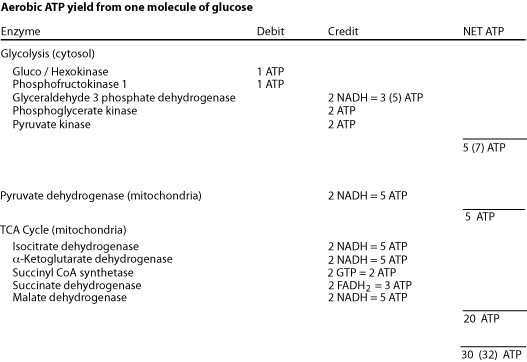
The complete aerobic oxidation of one molecule of glucose yields a net gain of 30 ATP or 32 ATP, depending on which carrier system transfers electrons from cytosolic NADH into the mitochondria.
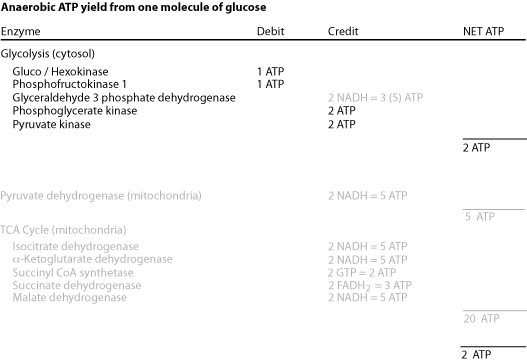
The incomplete anaerobic oxidation of one molecule of glucose yields a net gain of only 2 ATP because in the absence of oxygen the TCA cycle halts due to a deficit of the acceptor — oxygen — of the electrons from NADH and FADH2. The electrons generated as NADH by glycolysis in the cytosol are not transferred into the mitochondria, but instead are donated to pyruvate to form lactate — fermentation.
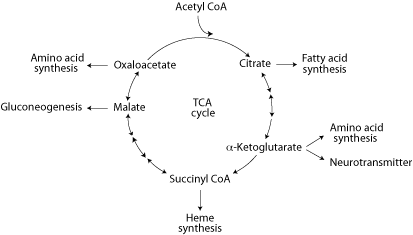
Intermediates of the TCA cycle are precursors for different biosynthetic pathways in different cell types. Because the liver is the site of synthesis of many biological molecules there is a high efflux of intermediates from the cycle. After a high carbohydrate meal citrate efflux and cleavage to acetyl CoA provides acetyl units for cytosolic fatty acid synthesis. During fasting, gluconeogenic precursors are converted to malate, which exits the mitochondria for cytosolic gluconeogenesis. The liver also uses TCA cycle intermediates to synthesize some non-essential amino acids, and succinyl CoA is used in both liver and bone marrow for the synthesis of heme. In the brain
Removal of any of the TCA cycle intermediates depletes the four-carbons that regenerate oxaloacetate at each turn of the cycle, diminishing the ability of the cycle to oxidize acetyl CoA. For the TCA cycle to continue functioning, enough four-carbon intermediates must be supplied from the degradation of carbohydrates or certain other molecules to compensate for the depletion. Reactions that replenish TCA cycle intermediates are called “anaplerotic”, meaning “to fill up”.
Pyruvate carboxylase (reaction #29) is a major anaplerotic enzyme, which uses ATP to catalyze the addition of CO2 to three-carbon pyruvate, forming four-carbon oxaloacetate. Many tissues contain pyruvate carboxylase as an anapleoric enzyme, but its concentration is particularly high in the liver and the kidney cortex, where gluconeogenesis withdraws four-carbon oxaloacetate from the TCA cycle for the synthesis of glucose.
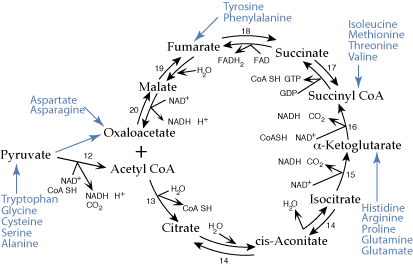
Eighteen common amino acids are glucogenic. Their carbon skeletons are transformed to oxaloacatate, some by reactions of the TCA cycle, and used to synthesize glucose. The other two common amino acids, Leucine and Lysine are ketogenic. Their carbon skeletons generate ketone bodies.
Eighteen of the common twenty amino acids degrade to carbon skeletons that enter the TCA cycle as four- or five-carbon units that can be converted to oxaloacetate, an intermediate of gluconeogenesis. The liver is the major site of gluconeogenesis and also the major site of amino acid degradation. Amino acid degradation in the liver provides a major source of carbon skeletons for the synthesis of glucose.
Click The Image
Regulation Of The TCA Cycle:
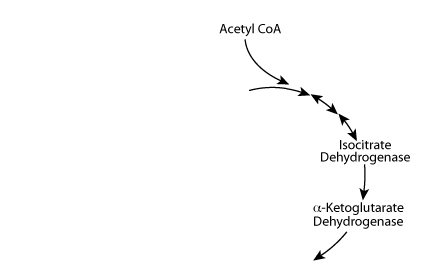
Acetyl CoA normally enters the TCA cycle.
Regulation Of The TCA Cycle:
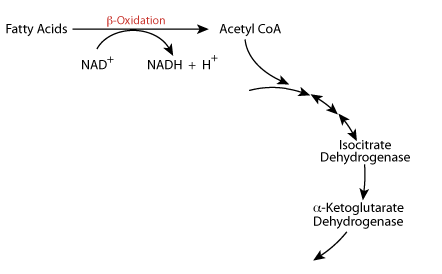
During Fasting / Starvation (Catabolic State) β-oxidation of fatty acids produces large amounts of NADH and large amounts of Acetyl CoA.
Regulation Of The TCA Cycle:
NADH Inhibits
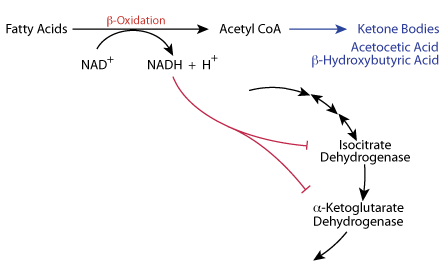
The NADH produced from fatty acid oxidation slows the TCA cycle at isocitrate dehydrogenase and α-ketoglutarate dehydrogenase. In the liver the Acetyl CoA is directed to the synthesis of Ketone Bodies.
Regulation Of The TCA Cycle:
Calcium Stimulates
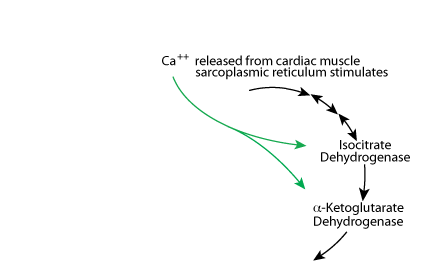
Calcium released from the sarcoplasmic reticulum increases the activity of isocitrate dehydrogenase and α-ketoglutarate dehydrogenase to increase energy production for cardiac muscle contraction.
The TCA cycle is regulated at isocitrate dehydrogenase and α-ketoglutatate dehydrogenase. Increased β-oxidation of fatty acids, which occurs during fasting, yields a large amount of NADH and Acetyl Coal. The increased NADH slows the TCA cycle at isocitric dehydrogenase and α ketoglutarate dehydrogenase. In the liver, the Acetyl CoA is directed toward the synthesis of ketone bodies; the liver is the only tissue that syntheses ketone bodies. In cardiac muscle, Ca2+ increases the activity of the TCA cycle to increase the production of energy for cardiac muscle contractions.