TETRAHYDROFOLATE & THE FOLATE ONE-CARBON POOL
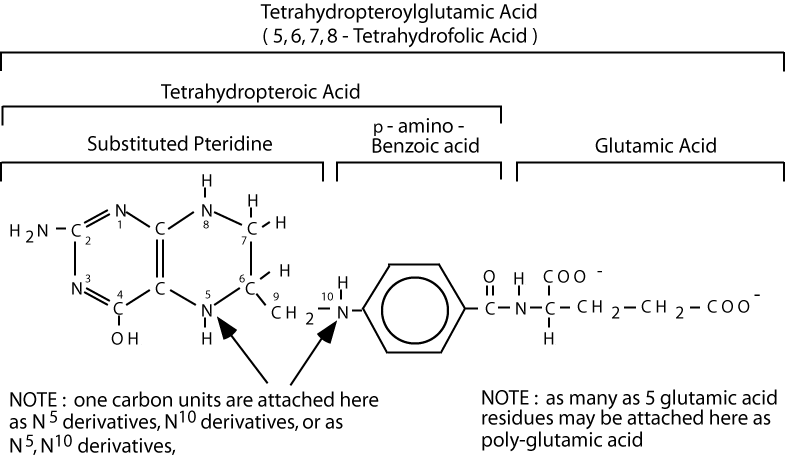
Folic acid (folate) is a vitamin that humans must take in their diet, as they cannot synthesize it. Bacteria and higher plants can synthesize folate from the bicyclic pteridine ring, p-aminobenzoic acid, and glutamate.
Sulfa drugs, which are used to treat certain bacterial infections, are analogues of p-aminobenzoic acid. They prevent bacterial growth and cell division by interfering with folate synthesis. Because human cells don’t synthesize folate, certain sulfa drugs can be used to treat some bacterial infections without significantly affecting folate levels in human cells because bacteria must synthesize folate, as they cannot uptake folate from their environment.

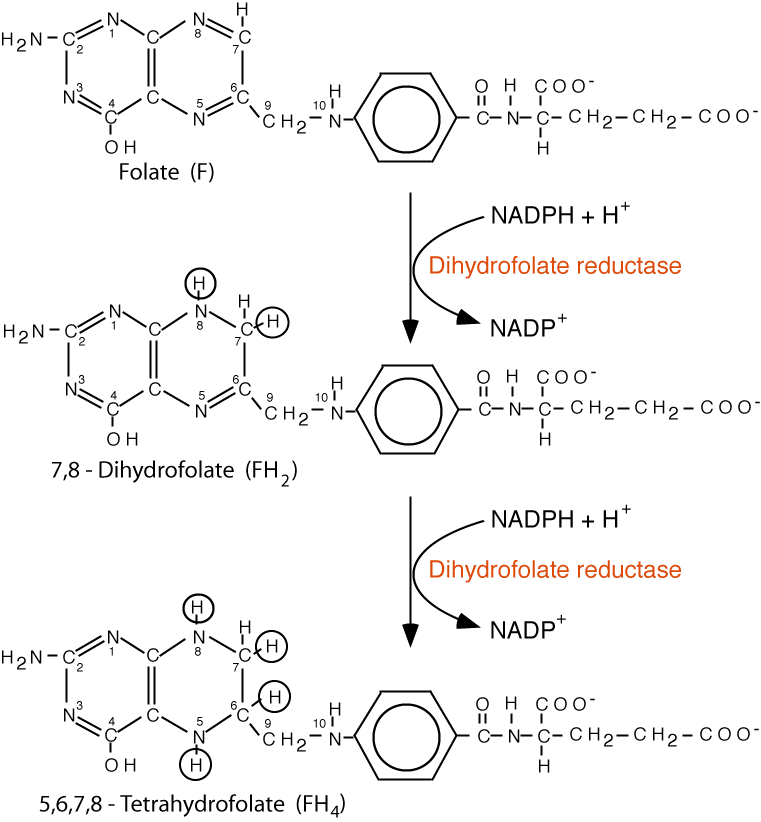
Tetrahydrofolate (FH4) is the active form. Dihydrofolate Reductase reduces folate to yield, first, 7,8-dihydrofolate (FH2), and then by a second reduction, to yield 5,6,7,8-tetrahydrofolate (FH4), also known as tetrahydropteroylglutamic acid. NADPH is the source of electrons for both reduction steps.
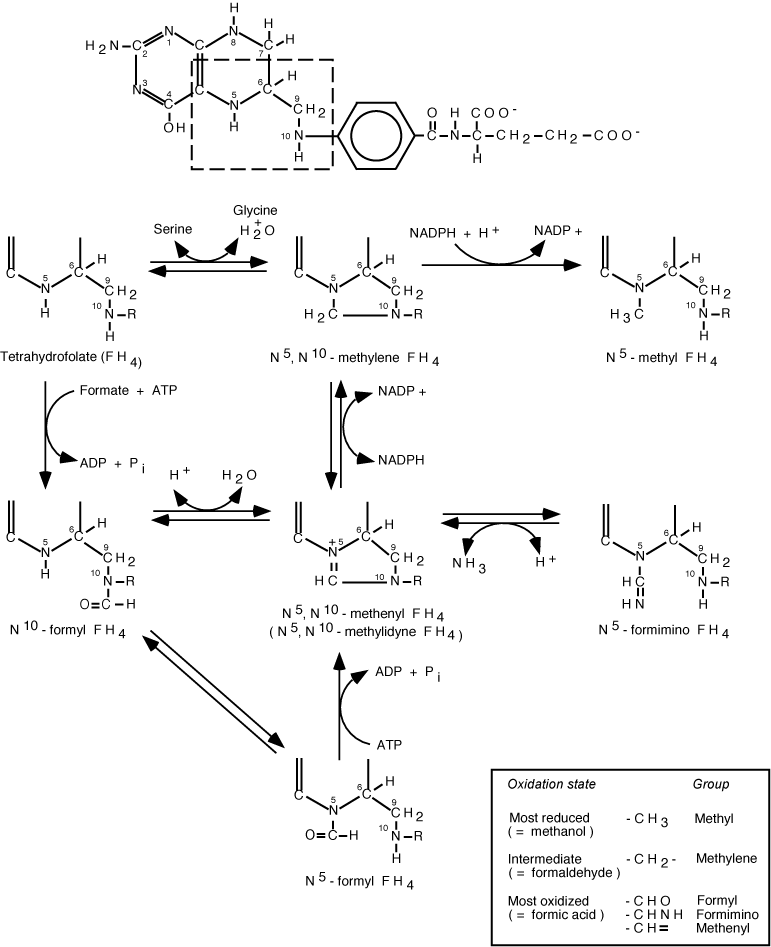
One-carbon units are attached either to nitrogen N5 or N10 or they form a bridge between N5 and N10.
Serine is the major source of one-carbon units donated to FH4. Because serine can be synthesized from glucose, dietary carbohydrate serves as a major source of carbon for the folate one-carbon pool.
Other one-carbon donors are glycine, which along with serine and formaldehyde (from, for example, epinephrine and choline breakdown), yield N5, N10-methylene FH4, histidine, which yields N5, N10-methenyl FH4 via the intermediates N-formiminoglutamate and N5-forminino FH4, and tryptophan, which donates a formate group during breakdown, to yield N10-formyl FH4.
While attached to FH4 the one-carbon units are oxidized and reduced, allowing one-carbon units to be accepted and donated in different oxidative states, as required by individual biochemical reactions.
Acceptors of one-carbon units from FH4 compounds include (but are not limited to) glycine from N5, N10-methylene FH4 to yield serine, the dTMP precursor deoxyuridine monophosphate from N5, N10-methylene FH4, to yield deoxythymidine monophosphate (dTMP), purine precursors, which derive carbon 2 and carbon 8 from N10-formyl FH4, and vitamin B12, which accepts a methyl group from N5-methyl FH4 that it subsequently donates in a reaction catalyzed by methionine synthase to remethylate homocysteine to methionine (one of only two reactions in the human body that require vitamin B12; see “Synthesis & Degradation > Methionine” in the top menu). [Note: the other reaction that requires vitamin B12 is catalyzed by methylmalonyl CoA mutase in the conversion of propionyl CoA generated by the degradation of several amino acids and in the β-oxidation of odd-chain fatty acids to succinyl CoA. When B12 is deficient, L-methylmalonyl CoA is not readily converted to succinyl CoA, and methylmalonic acid, a neurotoxin, accumulates in the blood (methylmalonic acedemia) and is excreted in the urine (methylmalonic acidurea).]
Structures of One-carbon Derivatives of FH4
The Folate Methyl Trap Theory
Click The Image
Interrelationships Of Folate One-Carbon Oxidation StatesB12N5
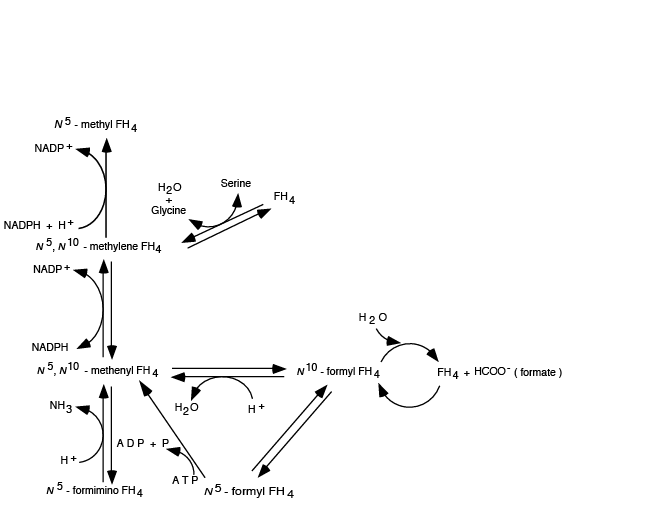
Abbreviated scheme of the relationships between FH4 oxidation states. All oxidation states of the attached one-carbon units can be reached from any single oxidation state due to the reversibility of the reactions, with the EXCEPTION that the reduction of N5, N10-methylene FH4 to N5 methyl FH4 is irreversible. Of all the oxidation states, N5 methyl FH4 is the most stable.
Vitamin B12 Accepts a Methyl Group From N5-methyl FH4
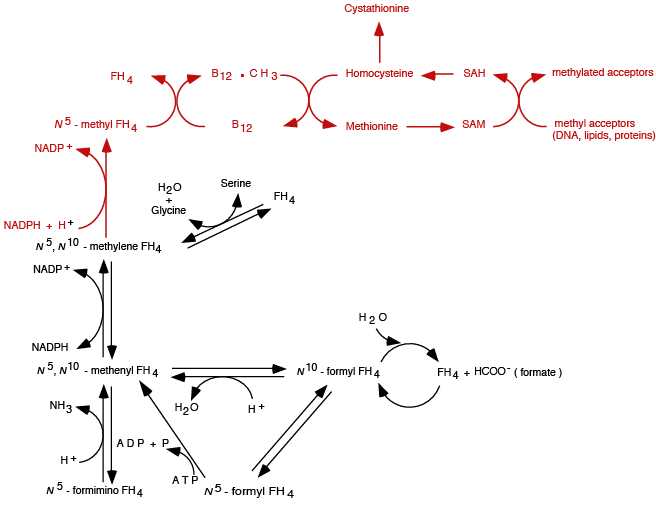
N5 methyl FH4 donates its methyl group to vitamin B 12, and vitamin B12 donates the methyl group to homocystein in the Methionine Synthase (Remethylation) reaction (see Synthesis & Degradation > Methionine in the top menu).
Vitamin B12 Accepts a Methyl Group From N5-methyl FH4
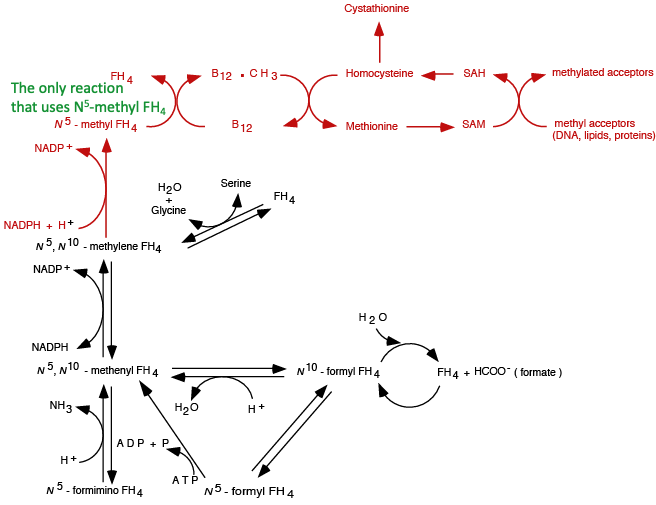
The ONLY reaction in the human body in which N5 methyl FH4 donates its methyl group.
Vitamin B12 DeficiencyN5
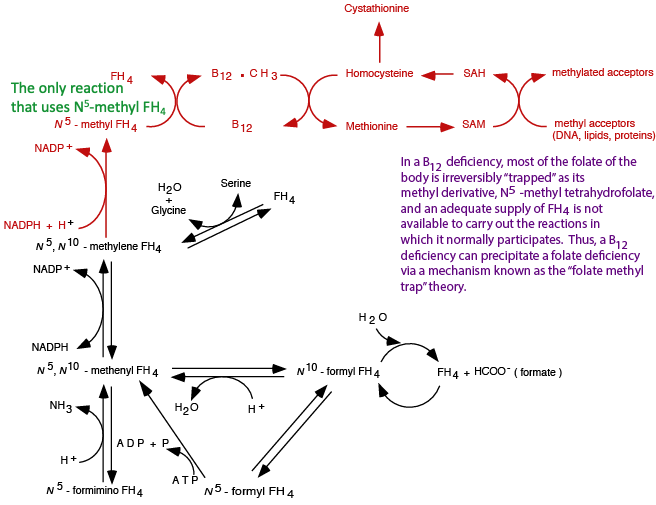
In a vitamin B12 deficiency, N5 methyl FH4 is unable to donate its methyl group, and because the reduction of N5, N10-methylene FH4 to N5 methyl FH4 is irreversible, N5 methyl FH4 accumulates, “trapping” FH4 as the N5 methyl FH4 derivative and depleting the other oxidation states, which are required for other essential reactions.
DNA Synthesis InhibitedB12N5
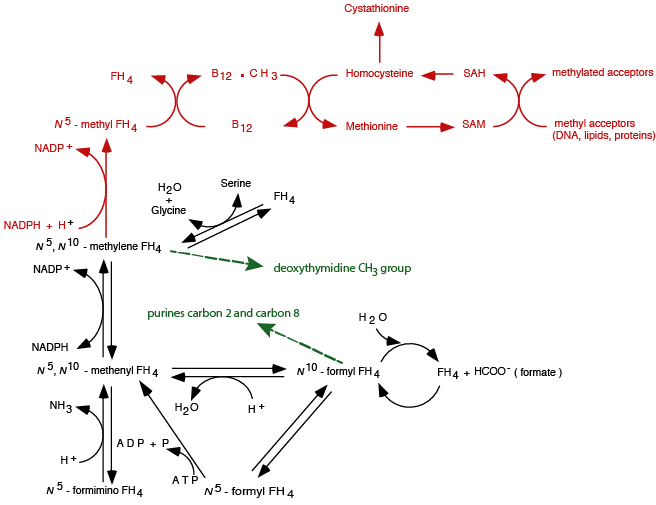
N5, N10-methylene FH4 donates its methylene group in a reaction that also reduces the methylene to a methyl group in the synthesis of deoxythymidine monophosphate, a precursor to deoxythymidine triphosphate. N10-formyl FH4 donates its formyl group to yield carbons 2 and 8 of the purine ring. If these folate one-carbon pool elements are depleted, either because of a folate deficiency or because in a B12 deficiency all of the folate is “trapped” as N5-methyl FH4, DNA synthesis halts for the lack of substrates.
Summary of the Effect of a B12 Deficiency on the Folate One-carbon Pool
- Vitamin B12 is obtained in small amounts from intestinal bacteria, but mainly in the diet from meats, eggs, dairy products, fish, poultry and seafood. Animals that serve as a source of B12 obtain it mainly from bacteria in their food supply.
- Deficiencies of vitamin B12 trap tetrahydrofolate as N5-methyl FH4.
- Reduction of N5, N10-methylene FH4 to N5-methyl FH4 by NADPH + H+ is irreversible.
- N5-methyl FH4 donates its methyl group in only one reaction in the body — the methylation of B12
- Deficiencies of vitamin B12 slow or prevent donation of the methyl group from N5-methyl FH4, thereby preventing regeneration of FH4.
- All, or almost all tetrahydrofolate accumulates as N5-methyl FH4, thereby diminishing or eliminating the tetrahydrofolate pool available for other reactions that require it.
- RESULT: A B12 deficiency causes an apparent folate deficiency, because all or almost all the folate is “trapped” as the N5-methyl FH4 derivative; a patient may present with symptoms of folate deficiency, when, in fact, he/she has a B12 deficiency. Appropriate tests must be done to distinguish a B12 deficiency from a folate deficiency.
Anti-folate Drugs
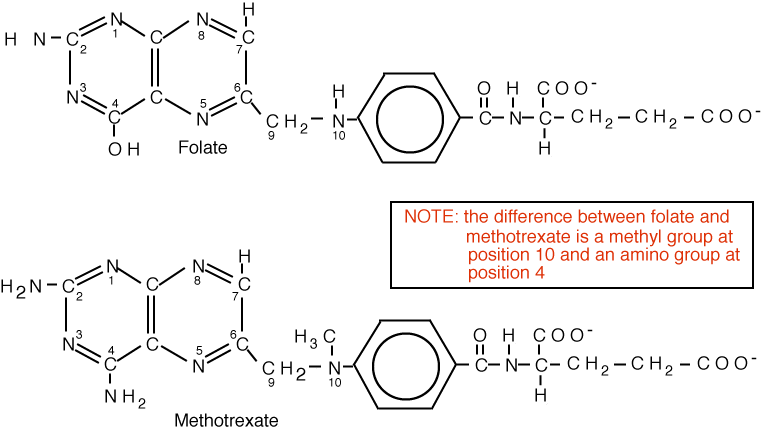
The antifolates were the first class of antimetabolites to enter the clinics 65 years ago. Their mechanism of action is due to the disruption of the metabolic pathways that require one-carbon moieties supplied by the B9 folate vitamins, which they resemble. While renewing tissues of the bone marrow and intestinal tract are also folate-dependent and are sites of antifolate toxicity, the clinical utility of antifolates was established with the identification of doses and schedules of administration that provided sufficient selectivity to make these drugs effective in the treatment of cancer as well as inflammatory disorders. A folate analog, methotrexate, is an anti-tumor, chemotherapeutic agent that acts by inhibiting dihydrofolate reductase.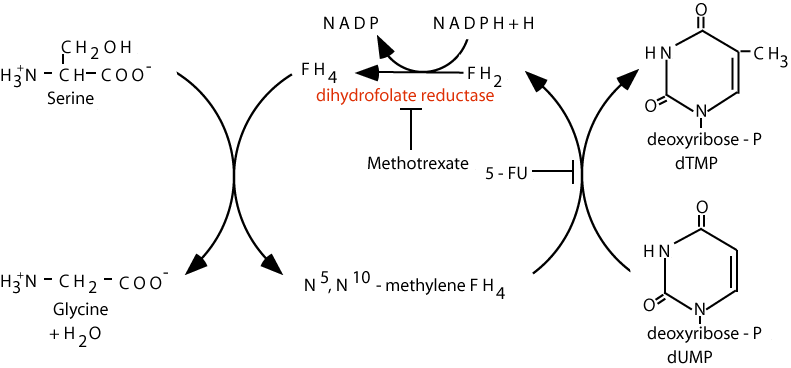
Deoxythymidine monophosphate (dTMP) is synthesized by the addition of a methyl group to deoxyuridine monophosphate (dUMP). dTMP is subsequently phosphorylated to the triphosphate form (dTTP) and used for DNA synthesis. N5, N10-methylene FH4 is the methyl group donor, and becomes oxidized to FH2 during the reaction (the methylene group is reduced to a methyl group for donation to dUMP to produce dTMP). The resulting FH2 must then be re-reduced by the enzyme dihydrofolate reductase before it can participate in subsequent methyl acceptor / methyl donor function.
Methotrexate, an anti-folate, inhibits dihydrofolate reductase, thereby starving cells of FH4. Cells starved of FH4 are unable to carry out reactions that require or produce folate derivatives, particularly the synthesis of dTMP and the purine ring, and their more highly phosphorylated forms required for DNA synthesis; without them DNA synthesis halts.
All cells undergoing cell division, and therefore requiring DNA synthesis, are targets of the anti-folate, methotrexate. Because tumor cells divide rapidly and incessantly, they are the prime target for killing, although other cells of the body that normally undergo continuous cell division, e.g. cells of the hair follicles, intestinal epithelium, cells of the immune system and male germ cells, are also targets, and killing those cells results in some of the side-effects of the drug. Normal cells, because of so-called “check points” in the cell cycle don’t divide if DNA synthesis does not proceed to completion. However, if DNA synthesis is prevented for a long period of time, they do die by undergoing apoptosis (programmed cell death). Tumor cells, however, have lost the cell cycle “check points” and continue to divide even though DNA synthesis has not completed successfully. As a result, they undergo so-called “mitotic catastrophe”, i.e., their daughter cells have less than the normal amount of DNA and, consequently die. This difference between normal and tumor cells is a central factor in the logic of anti-folate treatment to differentially kill tumor cells. Anti-folates are poisons; the logic is to use them in a regime that kills the tumor but not the patient!
A REVIEW OF ONE-CARBON TRANSFERS
Groups containing a single carbon atom can be transferred from one compound to another by different one-carbon “carrier” systems.
- Biotin transfers one-carbon units in the most oxidized form, as CO2. (For example, the enzymes pyruvate carboxylase and acetyl CoA carboxylase add CO2 to their respective substrates pyruvate and acetyl CoA and require biotin as the CO2 donor.)
- S-adenosyl methionine (SAM), tetrahydrofolate, and vitamin B12 transfer one-carbon units at oxidation levels lower than CO2.
- SAM donates the methyl group derived from methionine to several recipients at the methanol level of oxidation (CH3).
- Tetrahydrofolate, which is produced from the vitamin folate, obtains one-carbon units form serine, glycine, histidine, formaldehyde and formate. While attached to FH4 the one-carbon units undergo oxidation and reduction. Once reduced to the methyl level, however, the one-carbon unit cannot be re-oxidized (the “folate methyl trap”). Some major recipients of one-carbon units from FH4 are deoxyuridine monophosphate (dUMP) to form deoxythymidine monophosphate (dTMP), the amino acid glycine to form serine, the precursors of the purine bases to produce carbons C2 and C8 of the purine ring, and vitamin B12.
- B12 accepts a one-carbon unit from N5-methyl FH4 at the methanol level of oxidation (CH3), and donates it at the same level of oxidation (for example, in the methylation of homocysteine to form methionine).
Folate and Neural Tube Defects
Neural tube defects (NTDs) are common congenital malformations in humans, occurring at a frequency of almost one every 1000 live births in the United States population. The costs are enormous. In California alone, where studies were done, the number of children who are live-born with spina bifida each year generates over $60,000,000 in lifetime medical expenses. In the United Kingdom, NTDs account for 15% of perinatal deaths, second only to cardiac defects among congenital malformation-induced perinatal mortality.While etiologically heterogeneous, NTDs are, for the most part, multifactoral in their pathogenesis, having both genetic and environmental factors contributing to their development. Several factors have been suggested to influence NTD risk, including genetic predisposition, maternal obesity, and maternal exposure to numerous exogenous agents during early pregnancy, including anticonvulsant drugs. However, periconceptual supplementation of the maternal diet with a multivitamin containing folic acid is the only identified factor that has been definitively shown to have a significant relationship to NTD. Initial efforts demonstrated that first trimester levels of several micronutrients, particularly folate, were significantly lower in mothers of NTD-affected infants than in mothers of healthy infants. Subsequent nonrandomized trials conducted among women who had previously given birth to an infant affected with anencephaly or spina bifida demonstrated that folic acid, or multivitamins taken in the periconceptional period, resulted in a 75% reduction in the recurrence risk for a NTD. This observation has been verified in a double-blind, placebo-controlled, randomized study, which observed a 72% reduction in NTD recurrence risk when the maternal diet was supplemented with 4 mg. of folic acid per day. These studies strongly support the hypothesis that periconceptional supplementation of the maternal diet with folic acid in the dose range of 0.4 to 5 mg. per day is sufficient to overcome the majority of NTD recurrent risk.
Recent evidence suggests that supplementation with multivitamins containing folic acid reduces the occurrence risk for NTDs, just as it reduces the recurrence risk. In one study 0.8 mg. of folic acid taken daily significantly reduced a woman’s risk of having an infant with a NTD, supporting a previous study showing supplemental folate reduced, by 60%, the incidence of NTDs. A third study showed a 72% NTD risk reduction among women whose folate intake from both dietary and supplemental sources in early pregnancy exceeded 0.35 mg. per day, compared to women whose intake of folates was less than 0.18 mg. per day. Several other studies corroborate these findings. Women taking the higher dose of 4 mg. per day should do so only under medical supervision, as this higher dose can obscure the signs and symptoms of vitamin B12 deficiency. Folate's potential to reduce the risk of neural tube defects is so important that the Food and Drug Administration requires food manufacturers to fortify enriched grain products with folic acid.