AMINO ACID NITROGEN
After a meal that contains protein, amino acids released by digestion pass from the gut through the hepatic portal vein to the liver. In a normal diet containing 60 - 100 grams of protein, most of the amino acids are used for the synthesis of proteins in the liver and in other tissues. Carbon skeletons of excess amino acids may be oxidized for energy, converted to fatty acids, or, in some physiological situations, converted to glucose.During fasting, muscle protein is cleaved to amino acids, some of which are partially oxidized to produce energy. Portions of these amino acids are converted to alanine and glutamine, which, along with other amino acids are released into the blood. Glutamine is oxidized by various tissues, including the gut and kidney, which convert some of its carbons and nitrogen to alanine. Alanine and other amino acids travel to the liver, where the carbons are converted to glucose and ketone bodies and the nitrogen is converted to urea, which is excreted by the kidneys. Several enzymes are important in the process of interconverting amino acids and in removing nitrogen so that the carbon skeletons can be utilized. These include transaminases, glutamate dehydrogenase and deaminases. Because reactions catalyzed by transaminases and glutamate dehydrogenase are reversible, they can supply amino groups for the synthesis of non-essential amino acids.
-
Transamination is the major process for removing nitrogen from amino acids.
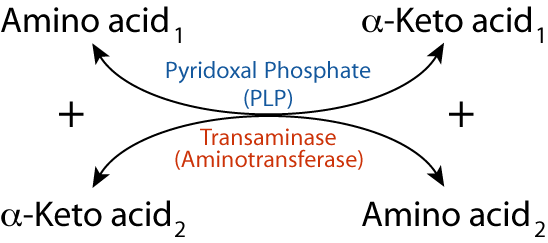
- transfer of an amino group from one amino acid (which is converted to its cognate α-keto acid) to another α-keto acid (which is converted to its cognate α-amino acid) by Transaminase (aminotransferase). The nitrogen from one amino acid thus appears in another amino acid
- one of the α-keto acid / α-amino acid pairs is always α-ketoglutarate / glutamate
-
EXAMPLE: the amino acid aspartate can be transaminated to form its cognate α-keto acid oxaloacetate. The amino group of aspartate is transferred to α-ketoglutarate by the enzyme aspartate transaminase (aminotransferase).
Aspartate Transaminase
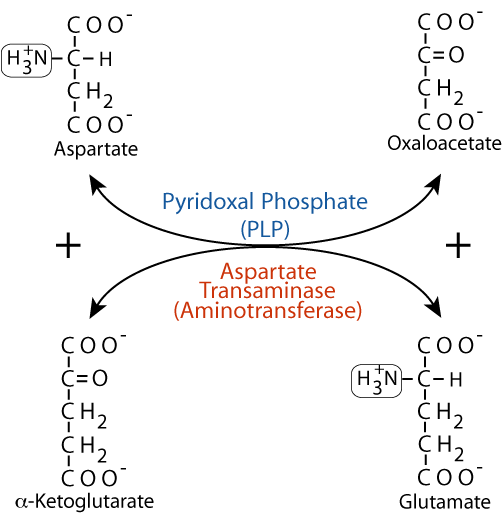
- Transaminases exist for all amino acids except lysine and threonine.
- Different transaminases recognize different amino acids, but they all use α-ketoglutarate and glutamate as one α-keto acid/α-amino acid pair. α-ketoglutarate and glutamate, therefore play a pivotal role in amino acid nitrogen metabolism.
- Pyridoxal phosphate (PLP), a derivative of vitamin B6 is a required cofactor for all transaminases.
- Because transamination reactions are reversible they can be used to remove nitrogen from amino acids or to transfer nitrogen to α-keto acids to form amino acids. They participate in both amino acid degradation and amino acid synthesis.
- Glutamate dehydrogenase catalyzes the oxidative deamination of glutamate.
- NH4+ released, α-ketoglutarate formed
-
NAD+ or NADP+ required
Glutamate Dehydrogenase
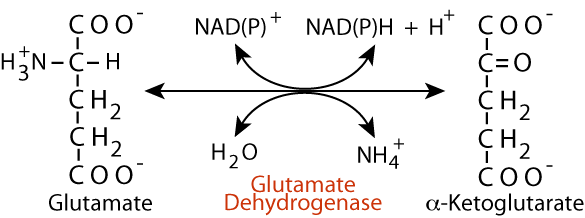
- reversible — one of only three reactions in humans that can “fix” NH4+, i.e., covalently link it to carbon by the reverse reductive amination of α-ketoglutarate
- in mitochondria of most cells
- Some amino acids for which transaminases exist release their nitrogen more frequently as NH4+
- Deamination by dehydration
- serine (enzyme = serine dehydratase; yields pyruvate + NH4+)
- threonine (enzyme = threonine dehydratase; yields α-keto butyrate + NH4+)
- Direct deamination
- histidine (enzyme = histidase; yields urocanate + NH4+)
- Hydrolytic deamination (uses water)
- asparagine (enzyme = asparaginase; yields aspartate + NH4+)
- glutamine (enzyme = glutaminase — releases the amide nitrogen; yields glutamate + NH4+)
- Methionine degradation yields free ammonium ion (NH4+)
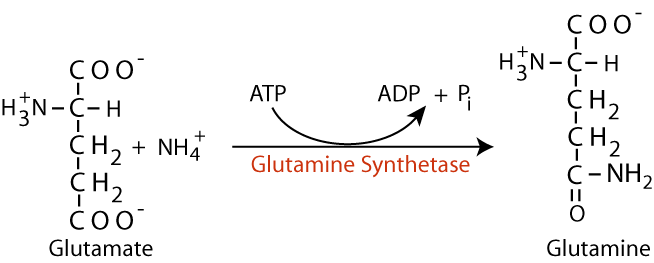
Glutamine Synthetase
- Deamination by dehydration
- Glutamine Synthetase
Glutamine is produced from glutamate by the addition of nitrogen to the glutamate γ carboxyl group by an ATP-dependent reaction catalyzed by Glutamine Synthetase — one of only three reactions in humans that can “fix” NH4+, i.e., covalently link it to carbon. - NH3 + H+<——> NH4+
At physiological pH NH4+ / NH3 = 100. However, NH3 can cross cell membranes, allowing, for example, NH3 to pass into the urine from kidney tubule cells to decrease the acidity of the urine by binding protons to form ammonium ions (NH4+). This is an important mechanism for maintaining normal pH, allowing excess proton excretion by providing a proton buffer; particularly important during acidosis (see “ AA Flux” in the top menu). The kidney uses glutamine, in particular, as a source of NH3 to buffer excess protons. - Summing up: The pivotal role of glutamate
- Accepting and donating ammonia (NH3) and ammonium ion (NH4+)
- α-ketoglutarate can collect nitrogen from other amino acids as a consequence of transamination reactions to yield glutamate.
- α-ketoglutarate can accept an amino group from NH4+ via the reversible glutamate dehydrogenase reaction to yield glutamate.
- The glutamate amino group may be released as NH3 -> NH4+ via the reversible glutamate dehydrogenase reaction or transferred to various α-keto acids.
- Glutamate can accept a second, amide, nitrogen on its γ carbon via the glutamine synthetase reaction to yield glutamine.
- Glutaminase releases the amide nitrogen from glutamine as ammonium ion (NH4+)
Click The Image
Trans-Deamination:
Synthesis Of Urea From Amino Acid Nitrogen In The Liver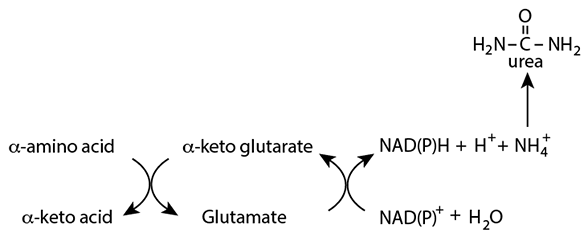
The liver, but not other tissues, synthesizes urea to dispose of nitrogen delivered to it by amino acids. Because ammonia (ammonium ion) is a neurotoxin its level in the blood must be kept relatively low, so it must be not be transported as such in the blood. Rather, almost all nitrogen is transported throughout the body as amino or amide groups of other molecules, particularly alanine and glutamine. In the liver, nitrogen destined for disposal is removed from amino acids in a two-step process referred to as “trans-deamination”.
Trans-Deamination:
Transamination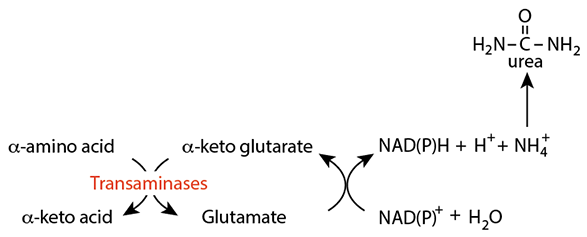
Transamination removes the amino group, leaving the amino acid carbon skeleton as an α-keto acid, which can be used for several purposes by the liver. α-ketoglutarate is the acceptor of the ammonia resulting in the formation of glutamate.
Trans-Deamination:
Deamination Of Glutamate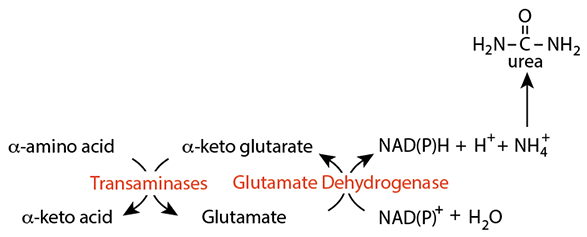
Glutamate dehydrogenase removes the alpha amino group from glutamate as ammonia (readily converted to ammonium ion), which the liver can use to synthesize urea for disposal. Urea is non-toxic and can be transported in the blood to the kidney for excretion.
Trans-Deamination:
Reversible Reactions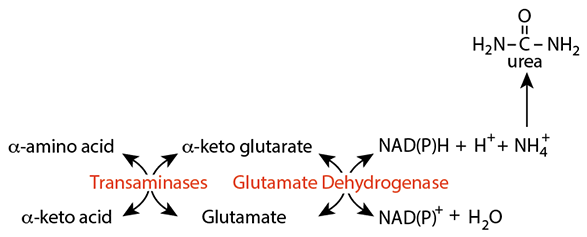
Because transaminases and glutamate dehydrogenase are reversible, they can remove ammonia (ammonium ion) from extra-hepatic tissues.
Trans-Deamination:
Glutamate Dehydrogenase In Extra-Hepatic Tissues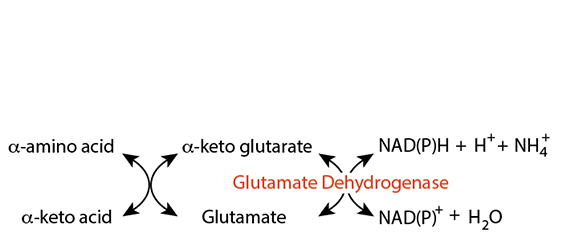
Glutamate dehydrogenase "fixes" nitrogen as an ammonium ion to form the α-amino group of glutamate. The ammonium ion could be generated from several amino acids or other nitrogen-containing compounds that undergo deamination in extra-hepatic tissues.
Trans-Deamination:
Transamination In Extra-Hepatic Tissues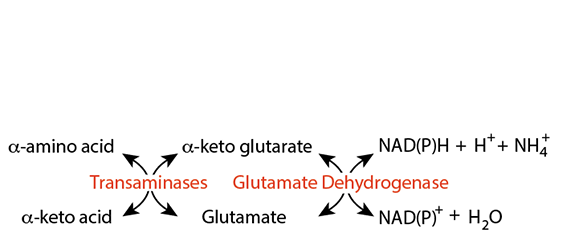
The glutamate α amino group is transferred to an α-keto acid by an appropriate transaminase. Pyruvate, because it is an abundant α-keto acid (probably the, or one of the most abundant α-keto acids in cells) gets the amino group via alanine transaminase (amino transferase). The amino group can then be safely transported between tissues because when attached to another molecule it is not a neurotoxin.
Trans-Deamination:
After Transport To The Liver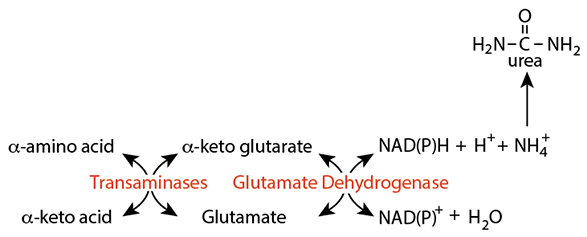
Nitrogen transported from extra-hepatic tissues to the liver as an amino acid can be released by trans-deamination for disposal in urea.
- NH4+, largely produced directly from glutamate by glutamate dehydrogenase, and aspartate (which may be produced by transamination of oxaloacetate, with glutamate as the amino group donor) provide nitrogen for urea synthesis by the urea cycle (see Nitrogen > Urea Cycle in the top menu) for elimination of nitrogen from the body in the urine.
- Accepting and donating ammonia (NH3) and ammonium ion (NH4+)
- Providing nitrogen for amino acid synthesis
- NH4+ + α-ketoglutarate <——> glutamate (enzyme = glutamate dehydrogenase)
- glutamate may transfer nitrogen by transamination reactions to α-keto acids to form the corresponding amino acids; the mechanism by which non-essential amino acids obtain their amino group (see Synthesis & Degradation in the top menu)